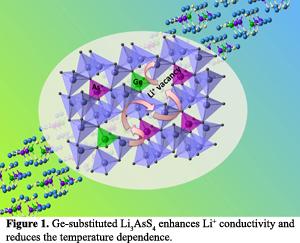
The substitution of Ge for As in Li3AsS4 results in an exceptionally stable ionic conductivity versus temperature, and enhances the ionic conductivity by two orders of magnitude. The performance of solid state batteries is dramatically sensitive to temperature due to the energy barrier associated with Li ion motion. To reduce the influence of temperature on the performance of solid state batteries, a low value of activation energy is highly desirable in practical applications. This research demonstrated that the Ge-substituted Li3AsS4 has the lowest activation energy among all known solid electrolytes. Furthermore, this study demonstrates that surface passivation of the solid electrolyte improves compatibility with metallic lithium electrodes. The combination of high ionic conductivity, low activation energy, and outstanding electrochemical stability makes the practical use of this electrolyte feasible for solid-state batteries with stable performance in a broad temperature range.
Gayatri Sahu, Ezhiylmurugan Rangasamy, Juchuan Li, Yan Chen, Ke An, Nancy Dudney, and Chengdu Liang, “A High-Conduction Ge Substituted Li3AsS4 Solid Electrolyte with Exceptional Low Activation Energy,” Journal of Materials Chemistry A, 2014. DOI: 10.1039/C4TA01243G
For more information

