Neutron and X-ray Scattering - Structures
Small Angle Scattering
Small and ultra-small angle neutron, X-ray and light scattering provide a means to accurately quantify pore and particle-size distributions over a wide range of scales (approximately 1 nm to 0.5 mm). Our ongoing work has shown how this can provide quantitative information on overall volumes, size distributions, core/rim structures, and surface and mass fractal properties of scatterers. Combinations of of SAS techniques can provide quantification of multiphase systems, and submillimeter mapping or large-area averages of changes in the above-mentions properties. When combined with Wide-angle techniques, localized phase identification is also possible. Figure * below shows and example comparing the cumulative porosities of sandstones and shales.
Neutron Spectroscopies – Dynamics
Inelastic and quasielastic neutron scattering (INS, QENS) and neutron spin echo spectroscopy (NSE) are applied to probe the diffusional dynamics and bond energetics of hydrogen-bearing species (e.g. water, nonaqueous electrolytes, hydrocarbons, surface functional groups, hydrous minerals), particularly at fluid-solid interfaces and in pore-confined fluids. These approaches are closely integrated with ab initio and classical molecular dynamics simulations (AIMD, CMD) to develop and validate the computational models and to use them to help interpret the experimental data. The image shows the direct comparison of water and OH vibrational and librational motions on hydrated SnO2 nanoparticle surfaces extracted from AIMD and INS (Wang et al., J. Phys. Chem. C 2014, 118, 10805).
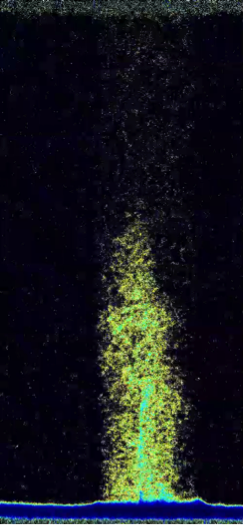
X-ray and Neutron Imaging (tomography, radiography)
We use X-ray and neutron tomography (3D) and the complementary radiographical (2D) imaging approaches to image the structure of porous media non-invasively, either in ex situ characterization measurements, or in situ observations of, for example, fluid transport or mineral growth or dissolution reactions. These techniques provide a powerful capability to develop a fundamental understanding of how macroscopic parameters such as porosity and permeability evolve in a system under a given set of conditions. Figure * below shows a single instance snapshot of water uptake into the Mancos shale. A combination of such images provides real-time, quantifiable movies of the process.
Multiscale Computational Modeling
Experimental studies of geochemical systems are complemented and integrated by molecular and coarse-grained models. We have developed a set of principles and computational techniques to refine these models based on experimental and ab initio reference data. The resulting models are being applied to the design of Gaussian charge polarizable models of aqueous electrolytes in contact with mineral surfaces and to multiscale problems involving adsorption of fluids in porous materials (Fig. A) and mineral dissolution or precipitation. Special rare-event simulation techniques, such as metadynamics, are used to determine the mechanisms of elementary dissolution steps, which serve as input to kinetic Monte Carlo simulations of mineral interfaces spanning large spatial and temporal scales, and aid in developing new rate expressions for mineral reactions.
Fluid-Cell Atomic Force Microscopy
Our laboratories are equipped with an Asylum instruments MFP-3D atomic force microscope. In addition to sample characterization, we use this instrument to observe the growth and dissolution of minerals in situ, at the micrometer scale. Coupled to our atomic-scale simulation, this technique provides a powerful way to understand, predict and control mineral growth and dissolution reactions.
High Pressure/Temperature Fluid Adsorption
Our group operates two Rubotherm high-pressure gravimetric excess sorption instruments. The 700 bar pure fluid system is used for the study of CO2 and light hydrocarbon adsorption to synthetic and natural geologic material under reservoir conditions. The temperature range is 10-120oC. Excess sorption data provide thermodynamic information about fluid-solid interactions and their dependence on pressure, temperature, and morphology and chemical composition of the substrate.

