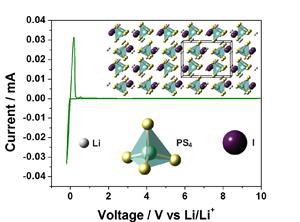
Coordination of iodine atoms within the Li3PS4 (LPS) electrolyte results in a new ceramic electrolyte with the formulation Li7P2S8I, a coordinated material between LPS and LiI. This new formulation takes advantage of the chemical stability of LiI to render an electrolyte with excellent compatibility with Li anode. Additionally, the iodine coordination within the crystal structure aids in circumventing oxidative instabilities at higher electrochemical potentials. As a result the new formulation delivers one of the best reported electrochemical stabilities for a solid electrolyte.
The electrolyte delivers outstanding performance with negligible kinetic limitations: namely, there is no observable resistance at the lithium/solid electrolyte interface. Low-temperature sintering of the material results in pinhole-free membranes. The low-temperature process can also facilitate fiber reinforcement of membranes for improved mechanical stability. The electrolyte has a demonstrated life of 800 cycles at ambient temperature with no measurable drop in performance. This work contributes to the development of advanced batteries that use lithium as the high-energy electrode.
E. Rangasamy, Z. Liu, M. Gobet, K. Pilar, G. Sahu, W. Zhou, H. Wu, S. Greenbaum, and C. Liang, “An Iodide-Based Li7P2S8I Superionic Conductor,” J. Am. Chem. Soc. 137, 1384 (2015). DOI: 10.1021/ja508723m
For more information

