
Filter News
Area of Research
News Topics
- (-) Microscopy (51)
- (-) Neutron Science (140)
- 3-D Printing/Advanced Manufacturing (128)
- Advanced Reactors (35)
- Artificial Intelligence (102)
- Big Data (62)
- Bioenergy (92)
- Biology (102)
- Biomedical (62)
- Biotechnology (24)
- Buildings (67)
- Chemical Sciences (74)
- Clean Water (31)
- Climate Change (106)
- Composites (30)
- Computer Science (199)
- Coronavirus (46)
- Critical Materials (29)
- Cybersecurity (35)
- Decarbonization (85)
- Education (5)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (112)
- Environment (201)
- Exascale Computing (44)
- Fossil Energy (6)
- Frontier (46)
- Fusion (59)
- Grid (67)
- High-Performance Computing (94)
- Hydropower (11)
- Irradiation (3)
- Isotopes (57)
- ITER (7)
- Machine Learning (51)
- Materials (150)
- Materials Science (149)
- Mathematics (10)
- Mercury (12)
- Microelectronics (4)
- Molten Salt (9)
- Nanotechnology (60)
- National Security (73)
- Net Zero (14)
- Nuclear Energy (111)
- Partnerships (51)
- Physics (64)
- Polymers (33)
- Quantum Computing (39)
- Quantum Science (73)
- Renewable Energy (2)
- Security (26)
- Simulation (53)
- Software (1)
- Space Exploration (25)
- Statistics (3)
- Summit (61)
- Sustainable Energy (130)
- Transformational Challenge Reactor (7)
- Transportation (99)
Media Contacts
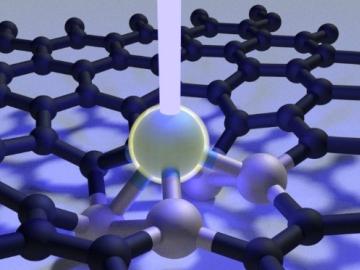
Scientists at Oak Ridge National Laboratory used a focused beam of electrons to stitch platinum-silicon molecules into graphene, marking the first deliberate insertion of artificial molecules into a graphene host matrix.
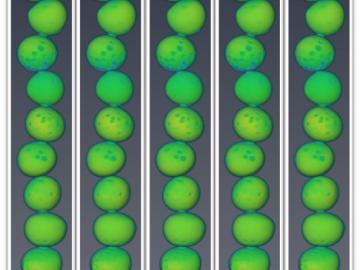
Oak Ridge National Laboratory researchers working on neutron imaging capabilities for nuclear materials have developed a process for seeing the inside of uranium particles – without cutting them open.
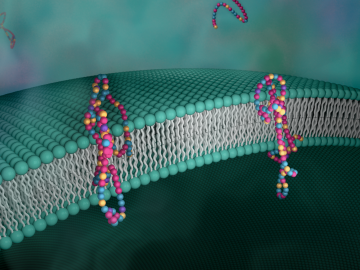
Biological membranes, such as the “walls” of most types of living cells, primarily consist of a double layer of lipids, or “lipid bilayer,” that forms the structure, and a variety of embedded and attached proteins with highly specialized functions, including proteins that rapidly and selectively transport ions and molecules in and out of the cell.
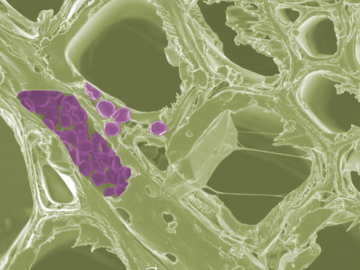
Scientists at the Department of Energy’s Oak Ridge National Laboratory have developed a new method to peer deep into the nanostructure of biomaterials without damaging the sample. This novel technique can confirm structural features in starch, a carbohydrate important in biofuel production.
Liam Collins was drawn to study physics to understand “hidden things” and honed his expertise in microscopy so that he could bring them to light.
Illustration of the optimized zeolite catalyst, or NbAlS-1, which enables a highly efficient chemical reaction to create butene, a renewable source of energy, without expending high amounts of energy for the conversion. Credit: Jill Hemman, Oak Ridge National Laboratory/U.S. Dept. of Energy
An international team of scientists, led by the University of Manchester, has developed a metal-organic framework, or MOF, material

Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory have new experimental evidence and a predictive theory that solves a long-standing materials science mystery: why certain crystalline materials shrink when heated.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.
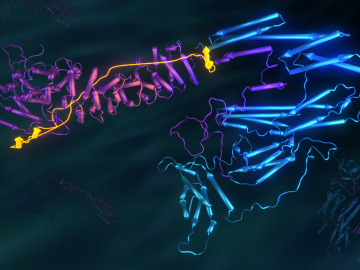
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source and High Flux Isotope Reactor to better understand how certain cells in human tissue bond together.


