PI: Takeshi Egami
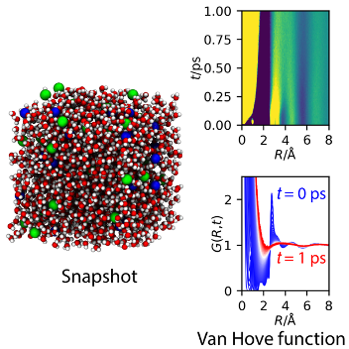
Scientists visualized correlated motions of water molecules and salt ions in real space through the Van Hove function. This observation is important because understanding the correlated motions of ions is a key to improving the mobility of ions in liquid electrolytes in many energy-storage devices.
Electrolytes are ubiquitous, but little is known about the dynamics of their water molecules and salt ions, and how they relate to macroscopic transport properties such as viscosity and electric conductivity. Conventionally, researchers analyze the data from inelastic X-ray or neutron scattering in reciprocal space and as a function of frequency, which makes it hard to identify the contribution from each component and their correlated dynamics.
Here, researchers used double Fourier transform on the results of high-resolution inelastic X-ray scattering to obtain the spatio-temporal atomic correlation function, a.k.a. the Van Hove function, of aqueous salt solutions. Utilizing the results of molecular dynamics simulation, they decomposed the Van Hove function into specific element–element correlations. By virtue of the capability of the Van Hove function to resolve dynamics as a function of distance and time, this work unambiguously identified the water–water and water–anion correlations. The results bridge the gap in knowledge between microscopic motions of ions and molecules and macroscopic transport properties.
Related publication:
Y. Shinohara, R. Matsumoto, M. W. Thompson, C. W. Ryu, W. Dmowski, T. Iwashita, D. Ishikawa, A. Q. R. Baron, P. T. Cummings, and T. Egami, “Identifying water–anion correlated motion in aqueous solutions through Van Hove functions,” J. Phys. Chem. Lett. 10, 7119 (2019); DOI: 10.1021/acs.jpclett.9b02891.


