Achievement:
Researchers from University of California Riverside, Drexel, and Oak Ridge National Laboratory (ORNL) identified the atomistic mechanism by which MXenes degrade in water. This oxidation results in a loss of desired properties. Using first-principles molecular dynamics calculations, the team found that water can directly react with MXene, resulting in MXene hydrolysis in ambient conditions, in support of recent experimental observations. The identified mechanism occurs rapidly and can occur even for defect free MXene. This result will help focus the development of mitigations to improve the stability of MXene in aqueous conditions. The team identified two potential approaches including control of the surface terminations and the use of negatively charged MXene.
Significance and Impact:
In the past decade, MXenes have quickly grown into a large family of two-dimensional (2D) carbides and nitrides. They have a general formula of Mn+1XnTx (n = 1, 2, 3 or 4), where M stands for a transition metal, X for carbon and/or nitrogen, and T for surface termination (−OH, −O, and/or −F). Because of their 2D morphology, hydrophilic surfaces, and metallic conductivity, MXenes have shown promise for a variety of applications, such as optoelectronic devices, supercapacitors, lithium-ion batteries, and sensors. However, chemical stability has been a major concern for wider adoption of MXenes because they tend to transform into oxides over time, resulting in structure degradation and loss of properties. Identifying the mechanism by which they degrade is an essential step to developing mitigations and facilitating eventual MXene-based technologies. Furthermore, we showed that negatively charging the surface or converting the surface O to −OH groups prevents close encounters of water molecules and the surface Ti atoms, thereby stabilizing the surface. This work highlights the importance of water as a key reactant in controlling the stability of MXenes.
Research Details
- Performed extensive first-principles density functional theory (DFT) based molecular dynamics for water intercalated MXenes.
- Identified that the water molecules attack the basal plane of Ti3C2O2 via irreversible adsorption on the surface Ti atom, followed by deprotonation and Ti pullout by the adsorbed −OH group with simultaneous Ti–C bond breaking.
- Identified two paths to stabilizing the MXene: negative charges and alternate surface terminations.
Facility: Calculations were performed at NERSC.
Sponsor/Funding: DOE BES
PI and affiliation: Paul Kent, Computational Chemistry and Nanomaterials Group, Computational Sciences and Engineering Division, ORNL
Team: Tao Wu and De-en Jiang (University of California Riverside), Paul Kent (ORNL), Yury Gogotsi (Drexel University)
Citation and DOI:
- Tao Wu, Paul R. C. Kent, Yury Gogotsi, and De-en Jiang, “How Water Attacks MXene”. Chem. Mater. 34 4975 (2022); DOI: 10.1021/acs.chemmater.2c00224
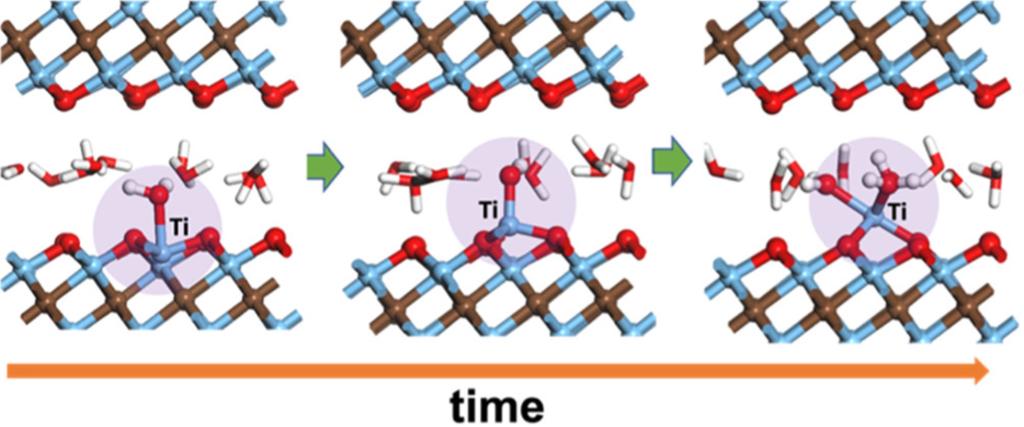
Summary: Two-dimensional (2D) transition metal carbides and nitrides (MXenes) have shown outstanding performances in electrochemical energy storage and many other applications. However, the stability of MXene remains a concern, especially its quick degradation in aqueous solutions under ambient conditions. Here, we report on the water/Ti3C2O2-MXene interfacial chemistry from first-principles molecular dynamics simulations at room temperature. Surprisingly, we find that the water molecules can attack the basal plane of Ti3C2O2 and pull the surface Ti atoms out, thereby reconstructing the surface. By tracking close encounters of water molecules and surface Ti atoms on the basal plane of Ti3C2O2, we show that the attack is initiated by the chemisorption of a water molecule on a surface Ti atom, followed by the breaking of Ti–C bonds and deprotonation of the water molecule, leading to the formation of Ti–OH on the Ti3C2O2 surface and a hydronium ion in the aqueous phase. Our finding highlights the susceptibility of Ti3C2O2 MXene to water attack, supporting recent experimental observations. Furthermore, we demonstrate that preventing close encounters of water molecules and the surface Ti atoms is key to the stability of the basal plane and can be realized by negatively charging the surface (thereby reorienting the O atoms of water away from the surface) or converting the surface O to −OH groups (thereby shifting the water layer further away from the surface). Our insights and approach highlight the importance of the reactivity of water when interfacing with 2D materials such as MXenes.


