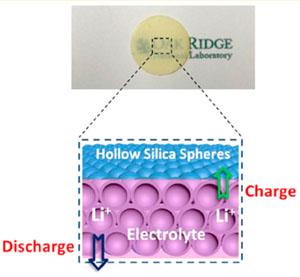
Hollow silica nanospheres were pressed into a film for dense packing. The nanospheres, which have microporous shells, confine electrolytes in their cavities, while the close-packed HS arrays block Li dendrites from forming and short-circuiting the cell during repeated charge−discharge cycling. After fully soaked with liquid electrolytes, HS films can achieve room-temperature conductivities as high as 2.5 mS/cm.
Furthermore, the HS-film scaffolds are broadly tunable. Different liquid electrolytes containing, for example, Na , Mg2 , or Al3 , can be chosen for the fabrication of other quasi-solid electrolytes, providing a promising alternative strategy for the development of next-generation rechargeable batteries.
Jinshui Zhang, Ying Bai, Xiao-Guang Sun, Yunchao Li, Bingkun Guo, Jihua Chen, Gabriel M. Veith, Dale K. Hensley, Mariappan Parans Paranthaman, John B. Goodenough, and Sheng Dai, “Superior Conductive Solid-like Electrolytes: Nanoconfining Liquids within the Hollow Structures,” Nano Letters (2015). DOI: 10.1021/acs.nanolett.5b00739.
For more information

