The next step in revolutionizing electric vehicle capacity
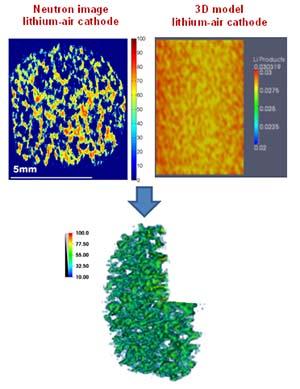
Using neutron-computed tomography, researchers at the CG-1D neutron imaging instrument at Oak Ridge National Laboratory’s High Flux Isotope Reactor (HFIR) have successfully mapped the three-dimensional spatial distribution of lithium products in electrochemically discharged lithium-air cathodes.
Lithium-air chemistry promises very high-energy density that, if successful, would revolutionize the world of electric vehicles by extending their range to 500 miles or more. The high-energy density comes from oxygen, the cathodic component, and the lithium-metal anode, which has close to an order of magnitude higher specific capacity than commonly used carbon anodes.
However, to become a workable technology, fundamental scientific issues need to be resolved. One major issue is deposition of various lithium decomposition products during the oxygen reduction reaction on the surface of the air-electrode. These products primarily originate from reduction of the electrolyte and solvent molecules during the discharge. The buildup of discharge products over time drastically reduces the surface electronic conductivity and affects the subsequent recharge.
Recent studies have found that the discharge reaction is strongly affected by electrolyte and solvent composition and is driven by complex reaction kinetics. Understanding the complex electrochemical and chemical decomposition of the electrolyte−solvent system and the resulting charge transfer kinetics at various current densities is therefore critical.
Neutron imaging research at HFIR revealed that there is a nonuniform distribution of the lithium products across the electrode thickness when they are discharged; the lithium concentration is higher near the edges of the lithium-air electrode and more uniform in the center. The origin of such anomalous behavior is the competition between the transport of lithium and oxygen and the accompanying electrochemical kinetics.
Quantitative understanding of these effects is a critical step toward rechargeability of lithium-air electrochemical systems. Improved spatial resolution of the neutron imaging technique combined with isotopic substitution methods will further enable scientists to understand spatial distribution of discharge product in the lithium-air cathodes.
Research Contacts: Hassina Bilheux, Jagjit Nanda, and S. Pannala
Published Work
Jagjit Nanda, Hassina Bilheux, Sophie Voisin, Gabriel M. Veith, Richard Archibald, Lakeisha Walker, Srikanth Allu, Nancy J. Dudney, and Sreekanth Pannala, “Anomalous Discharge Product Distribution in Lithium-Air Cathodes,” J. Phys. Chem. C 116, 8401−8408 (2012); DOI:dx.doi.org/10.1021/jp3016003.
Research at HFIR was funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences.

