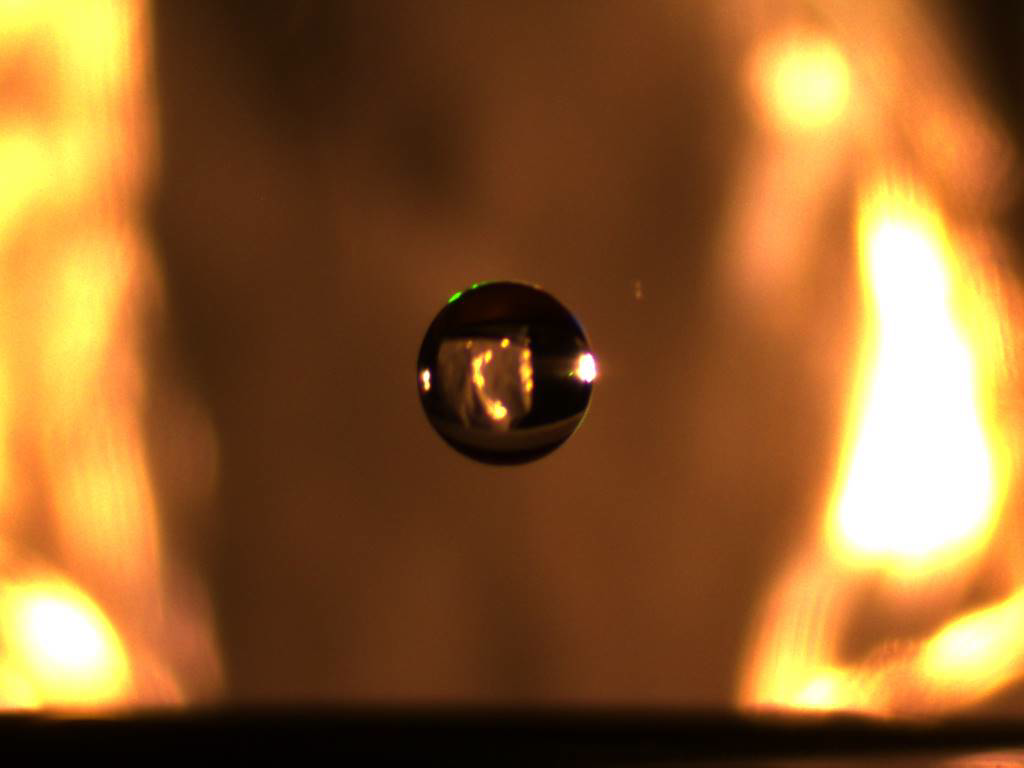
How do you get water to float in midair? With a WAND2, of course. But it’s hardly magic. In fact, it’s a scientific device used by scientists to study matter.
It’s called an electrostatic levitator. It uses carefully controlled electric fields to defy gravity by lifting charged particles and suspending them in midair. Levitators are especially useful in studying reactive materials, like high-temperature liquids such as molten metal, or complex processes like crystallization, letting researchers observe how atomic building blocks assemble to form new substances.
Recently, a group of researchers from Iowa State University successfully installed and tested a prototype electrostatic levitator for studying solutions at the High Flux Isotope Reactor’s WAND2 instrument, located at the Department of Energy’s Oak Ridge National Laboratory. The reactor’s core emits beams of neutrons to scientific instruments. As neutrons pass through experimental samples, they reveal how the material’s atoms are arranged and how they behave.
There are different kinds of levitators. Some use electromagnets and some even use soundwaves. Levitators allow researchers to study various types of materials without the need for cans or pedestals, which are used to mount samples but can often interfere with measurements as the neutrons scatter off the sample.
Scientists at HFIR and its sister facility, the Spallation Neutron Source, have deployed several types of levitators for different kinds of experiments, but none could study liquified solutions under a controlled atmosphere. The prototype is a special design that, prior to its installation at WAND2, had never been used for neutron scattering.
“This experiment allowed us to test the limits of neutron scattering just by using a small droplet and an even smaller amount of salt. We were also able to learn several lessons, such as how to improve signal over background interference and how to optimize data acquisition,” said ORNL instrument scientist Matthias Frontzek. “This experiment demonstrated the device’s feasibility and laid the foundation for future levitator experiments on WAND2.”
To test the levitator, the team used a droplet of water mixed with salt. After successfully levitating the sample, the team observed the suspended droplet as the water in the sample slowly evaporated over 4 1/2 hours, making the salt extremely concentrated. The researchers were able to capture striking images of the sample as it crystallized, recording the entire process with neutrons. The team hopes to learn more about what happens in solutions right before and during crystallization. The research could help spur advances in pharmaceutical and geochemistry studies, for example, offering a better understanding of how to grow and restore a coral reef.
“The test was a big success,” said ORNL’s Dante Quirinale, a development specialist who designs equipment for neutron scattering experiments. “It was the first time this type of solution levitator has been used for neutron scattering, and the tests show that we can build more advanced versions that can be used on WAND2 and other instruments as well. These levitators will enable us to do new types of experiments that weren’t possible before and help us broaden the neutron user community.”
The research was led by Iowa State Professor John Jonghyun Lee. His students included Brayden Berg, Gilbeom Seo, Yonghwa Yi, and Sai Katamreddy. The research is supported by the National Science Foundation (EPSCoR RII Track-4 2132131).
“We are studying complex nucleation by identifying the structural evolution of extremely saturated salt solutions which can only be done without the use of a container,” said Lee. “We can also use this experimental setup we’ve developed to study many other materials such as proteins, biominerals, colloids and polymers.”
HFIR and SNS are DOE Office of Science user facilities.
UT-Battelle manages ORNL for the Department of Energy’s Office of Science, the single largest supporter of basic research in the physical sciences in the United States. The Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.



