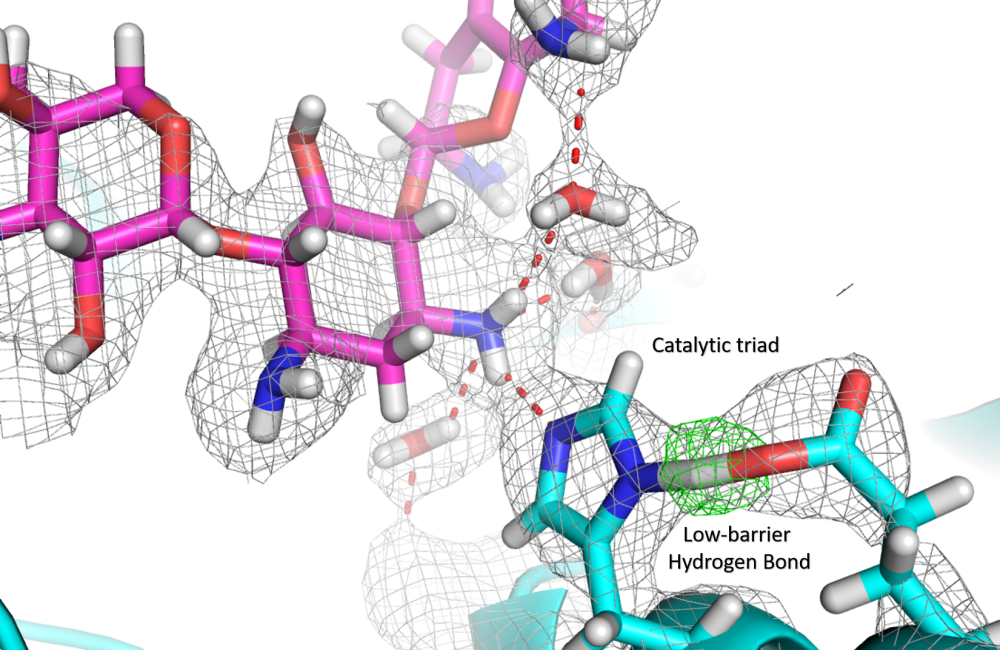
May 1, 2018 – An Oak Ridge National Laboratory-led team has observed how a prolific class of antibiotics may be losing its effectiveness as certain bacteria develop drug resistance by acquiring enzymes known as aminoglycoside modifying enzymes. Aminoglycosides are commonly used in antibiotics to treat tuberculosis, meningitis and listeriosis. Using X-rays and neutron diffraction, researchers found a known but previously undetected biological architecture called a catalytic triad within this enzyme group. The team identified a low-barrier hydrogen bond, involving a single hydrogen atom formed in this catalytic triad, which is crucial for the enzyme’s ability to disrupt the drugs’ molecular structure and render them ineffective to combat pathogenic bacteria. Detailed in Science Advances, this information provides new insights that could help improve future drug design.