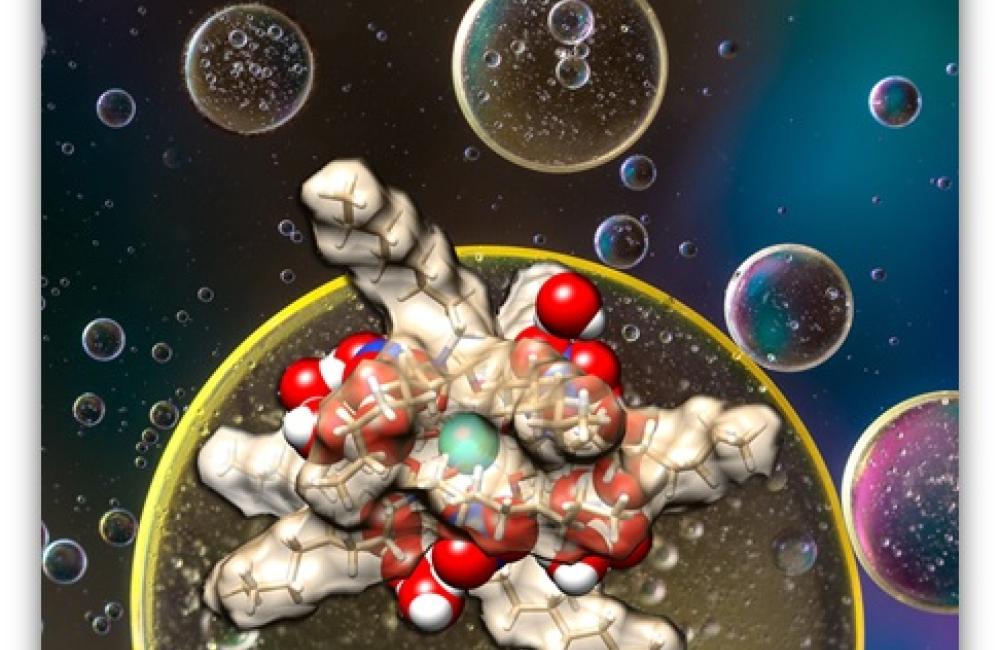
Peculiar outer-sphere water coordination of trivalent lanthanide complexes is shown in experiment and computation to correlate with the lanthanide selectivity of a diglycolamide ligand in the biphasic separation system.
Scientific Achievement
Correlation between lanthanide extraction and the amount of water coextracted in the organic phase is established and rationalized for the first time, indicating the importance of molecular interactions extending beyond the inner coordination shell
Significance and Impact
The findings have significant implications for the design of novel efficient separation systems and processes, emphasizing the importance of tuning both inner- and outer-sphere interactions to obtain total control over the lanthanide selectivity
Research Details
On the basis of the experimental and DFT studies, the mechanism underlying water uptake is related to the surface area of the nitrate counterions available to interact with coextracted water. MD simulations further elucidate that outer-sphere nitrate ions form hydrogen bonds with water molecules, which are dynamic in nature and can move easily around the lanthanide complex