Lower diesel emissions may be possible thanks to a catalyst that ‘stays young’
November 28, 2017 — Diesel vehicles today emit far fewer pollutants than older vehicles, thanks to a zeolite (hydrous silicate) catalytic converter that was invented around 10 years ago to reduce pollutants that cause the formation of acid rain and smog. Although many groups have investigated this catalyst, it remains unclear why a specific zeolite catalyst is much more effective than previous catalysts.
By managing to “see inside” the zeolite particles in three dimensions at the nanoscale, researchers from Utrecht University in the Netherlands and the Department of Energy’s Oak Ridge National Laboratory have been able to directly image phenomena responsible for their enhanced stability and durability.
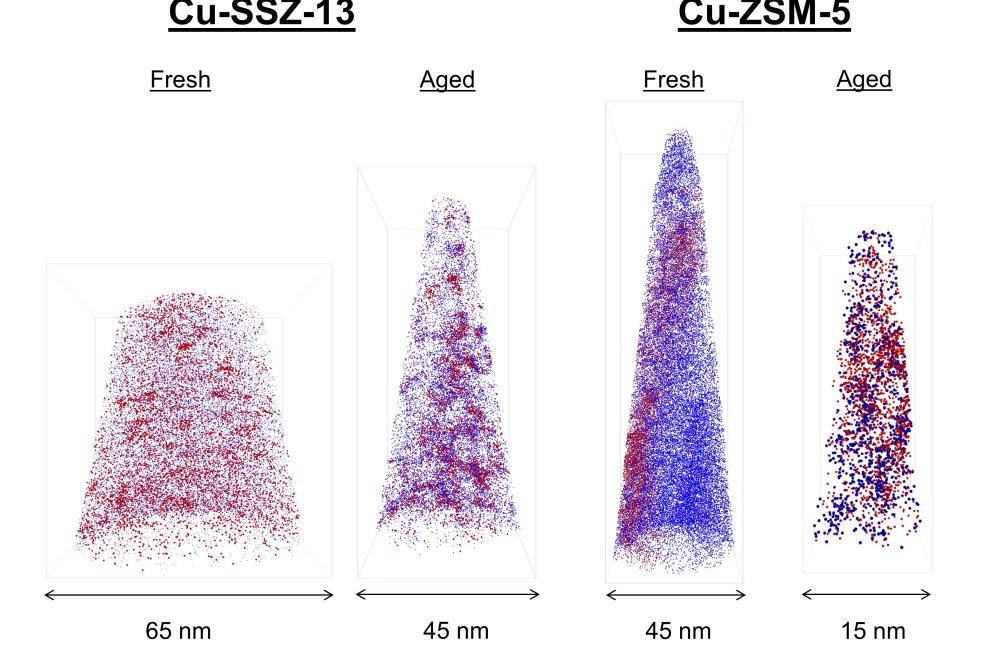
After simulating 135,000 miles of engine use, they compared a ‘new’ and an ‘aged’ version of the zeolite catalyst, which revealed that this catalyst retains much more of its original structure than other diesel catalyst formulations. The researchers also found the underlying reasons this zeolite catalyst is so much more stable over its lifespan and experiences only minimal damage. The results are published in Nature Communications.
Diesel catalytic converters are exposed to frequent temperature changes, extremely hot steam and pollutants, but they must remain stable for the entire life of the vehicle. The observed stability of this particular catalyst is due in part to its complexity.
“At first glance, zeolites may seem easy to understand, but the more you study them, the more fascinated you become by their complexity,” said Joel Schmidt of Utrecht University, lead author of the publication. “In this material, it is becoming more and more evident that the way its structure isolates the active reaction site is key to its stability, and advanced characterization methods that can help us understand the active catalyst site environment are vital to knowing the subtle, but important details of materials utilized in zeolite catalytic converters.”
Schmidt and his colleagues from Utrecht University connected with Jonathan Poplawsky at the Center for Nanophase Materials Sciences (CNMS), a DOE Office of Science User Facility at ORNL, to analyze the three-dimensional elemental distribution within the zeolite catalyst using a unique and powerful tool called local electrode atom probe tomography. With this technique, they could visualize all of the catalysts’ relevant chemical elements with three-dimensional resolution close to the atomic scale, for the as-produced “new” catalyst and after a 135,000-mile simulated aging procedure.
The researchers found that after aging, the zeolite catalyst exhibits enhanced stability compared with other diesel-vehicle catalysts due primarily to structural and chemical properties that prevent the formation of a deactivating copper-aluminum-oxide phase. Thus, the optimal nanoscale distribution of elements within the zeolite structure—which enables optimal cleaning of combustion byproducts—remains intact during aging.
“With this unique approach, we were able to add another piece to the puzzle of how to design catalysts that perform just as well at the end of a vehicle’s life as they did the day they rolled out of the factory,” said Bert Weckhuysen, Utrecht professor and co-author of the publication. “Since zeolite catalysts are used broadly in the chemical industry as well, insight on the migration of chemical elements under catalytic operating conditions is a very relevant contribution to realize more sustainable processes.”
This work is supported by the NWO Gravitation program, Netherlands Center for Multiscale Catalytic Energy Conversion, and the European Research Council. The atom probe tomography measurements were conducted at CNMS.
UT-Battelle manages ORNL for the DOE's Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit http://energy.gov/science/.
— Revised from a news release of Utrecht University.