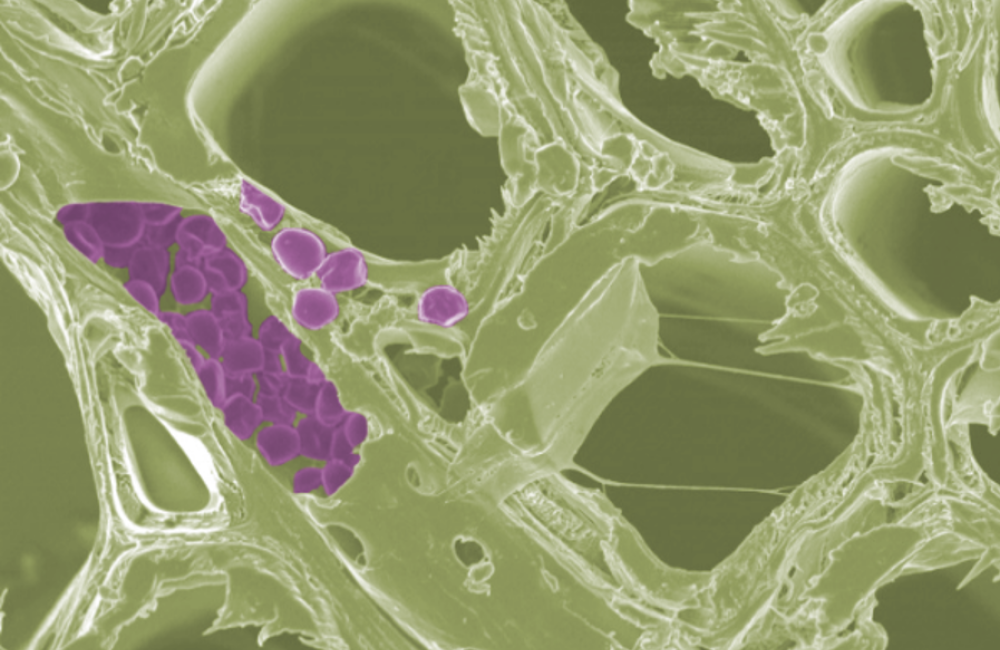
While exploring poplar cells using a scanning electron microscope, researchers observed sack-like structures, shown in purple, that were later identified as starch through Raman spectroscopy. Credit: Oak Ridge National Laboratory, U.S. Dept. of Energy; and CINaM, Aix Marseille University
Scientists at the Department of Energy’s Oak Ridge National Laboratory have developed a new method to peer deep into the nanostructure of biomaterials without damaging the sample. This novel technique can confirm structural features in starch, a carbohydrate important in biofuel production.
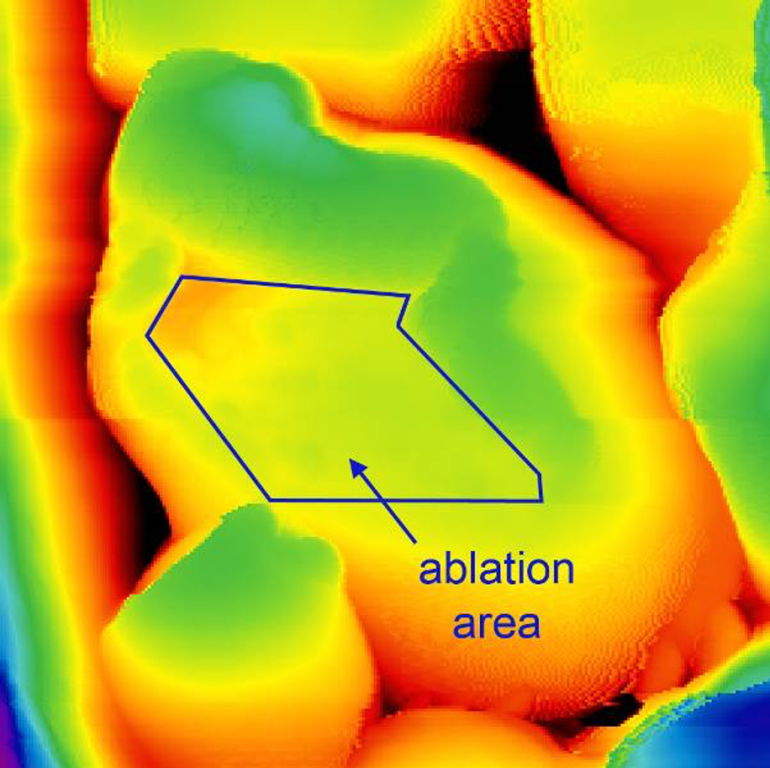
The research team used the sharp probe tip of an atomic force microscope, or AFM, to precisely punch tiny holes into a soft surface, such as a biological membrane, creating a detached layer that can be gently peeled away.
Using the new, nonintrusive soft mechanical nanoablation, or sMNA, technique, the team accessed starch granules without altering the nanostructure. Existing observation methods require damaging or destroying the starch’s outer layers, which can affect the physical properties of the granules.
“Our technique basically lifts the outer membrane,” said Ali Passian of ORNL’s Quantum Information Science group. “This leaves the interior structures almost untouched.”
As described in a paper published in the journal ACS Omega, sMNA allowed the team to observe interior properties of starch granules from stem samples of poplar trees.
In the United States, most biofuel comes from starch in corn kernels broken down into ethanol, but poplars have been longtime biofuel candidates because they grow quickly and produce lots of biomass. Though poplar biomass contains only 3 to 10% starch, a biological energy storage unit, the tree has abundant sugars wrapped up in polymeric materials such as cellulose, hemicellulose and lignin – important structural components that make up cell walls of tree trunks, branches and leaves.
Researchers want to learn more about the native, nanoscale properties of both starch granules and structural materials to grow productive poplars and best use them as biofuel feedstocks.
“Plant cell wall structure is really important if we’re going to go to the next generation of biofuels,” said Brian Davison, ORNL’s chief scientist for systems biology and biotechnology. “This study used starch as an example of how this technique can start to access some of these nanomechanical structural materials that we currently cannot observe in their original cellular surroundings.”
For this purpose, sMNA’s ability to study nanomechanical properties without damaging the sample offered advantages over traditional microscopy methods.
“At the level of polymers and ultrastructures of plant materials, slight chemical or physical changes can alter the measurement outcome, which makes the interpretation of the data more challenging.” Passian said. “This susceptibility to changes has motivated much research toward noninvasive and nondestructive measurement techniques.”
The research team used sMNA in conjunction with existing tools.
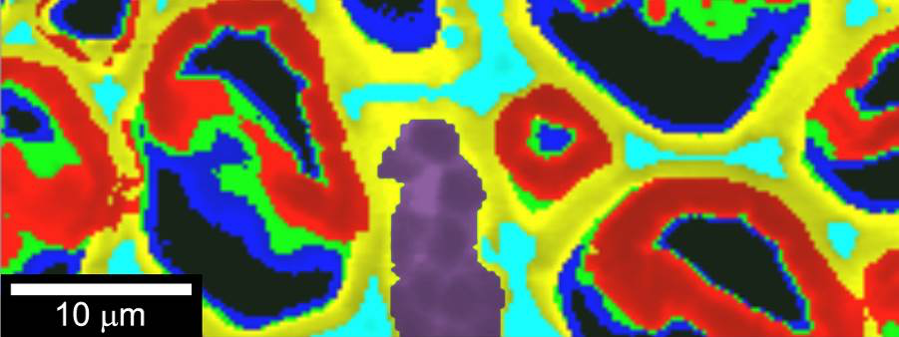
“To chemically confirm that the observed granules’ composition was indeed that of starch, we used Raman spectroscopy,” said ORNL’s Rubye Farahi, a co-author on the paper.
Next, the team deployed sMNA and used AFM to image repeating “blocklet” structures within the granules with unprecedented topographical detail revealing how the structures are spaced.
They also took internal and external measurements on the starch’s mechanical properties, including viscoplasticity, a measure of how a substance behaves when deformed, and Young’s modulus, a measure of a material’s stiffness.
“Those mechanical properties may help determine how starch granule structures might relate to the function of the rest of the plant,” Davison said, noting that he’d like to further use the technique to study more complicated structural materials in poplar.
The technique could also be applied to nonliving materials, according to Passian. He imagines it used on synthetic polymers or even 3D-printed materials.
“If we were able to apply it to this very delicate structure, others should be able to do the same with their samples of interest,” Passian said. “This opens up a range of possibilities in plant biology or any other areas that have a need for looking at delicate materials.”
ORNL collaborated with Aix Marseille University’s Centre Interdisciplinaire de Nanoscience de Marseille, or CINaM, on this work. Co-authors on the study titled, “Nanomechanics and Raman Spectroscopy of in Situ Native Carbohydrate Storage Granules for Enhancing Starch Quality and Lignocellulosic Biomass Production,” include Ali Passian, Rubye H. Farahi, Udaya C. Kalluri and Brian H. Davison of ORNL, and Aude L. Lereu and Anne M. Charrier of Aix Marseille University. ORNL and CINaM jointly produced images in this research.
This research was supported by the BioEnergy Science Center, now the Center for Bioenergy Innovation, or CBI, led by ORNL. CBI is one of four DOE Bioenergy Research Centers supported by the Department of Energy’s Office of Science created to expand on the foundational successes of former bioenergy research centers and to lay the scientific groundwork for a new robust, biobased economy.
UT-Battelle manages ORNL for DOE’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.