Project Details
Critical Materials Institute (CMI) 1.1.12 project is focused on developing economical methods of recovery of rare earths (RE) from traditional/nontraditional sources including ores, tailings, and processing streams leading to industry adoption of one or more CMI technologies and to expanded domestic sources of critical materials. Emphasis is on improving the economics of producing concentrates via design of selective collectors, improving state-of-the-art leaching efficiency, RE recovery from leachates, and separation of fine RE-containing solids from viscous liquids. Improving ore recovery by increasing flotation concentration grades makes the subsequent separation steps more efficient and economical, which could open up new commercially viable sources for trace minerals. However, any developments in ore beneficiation and the whole process of finding better collector molecules have been mostly empirical. The important question of why relatively small changes in the structure of the ligand makes large differences in mineral recovery and selectivity remains open. We addressed this question under the CMI 1.1.12 project with the aim to develop structure-properties relationships that would allow us to design more efficient and selective collector agents for RE recovery and co-producing critical materials. Employed tools include supercomputing, laser spectroscopy, X-ray fluorescence, electron microscopy, and a range of separation equipment such as flotation cells, leaching vessels, cyclones, centrifuges, filters, and extraction contactors.
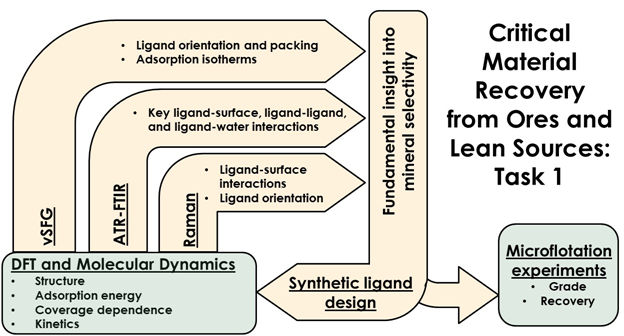
The rational basis for designing improved collectors includes several important components, as shown in the project logic diagram (Figure 1). First, density functional theory (DFT) calculations are used to calculate the most stable mineral surface structure and particle shapes. Next, the most stable surface compositions will be the targets for adsorption by ligands binding groups. Using the above information from DFT as a basis for structure-based design, the de novo molecular design approach can be employed to link functional groups on the surface and identify a number of viable ligand architectures. The final ligand evaluation and ranking is performed using DFT, which is a necessary step to accurately include the effects of water, water-ligand, and ligand-ligand interactions at the interface. Simulation results are compared with orientational and spectral analysis provided by surface sum frequency generation (SFG) vibrational spectroscopy and adsorption characteristics obtained from Fourier Transform Infrared (FTIR), Raman spectroscopy, and isothermal titration calorimetry (Alex Navrotsky group at ASU). New mechanistic insights obtained from a multifacted approach are used to rationalize the results of flotation experiments performed by the group of Corby Anderson at CSM.


