Project Details
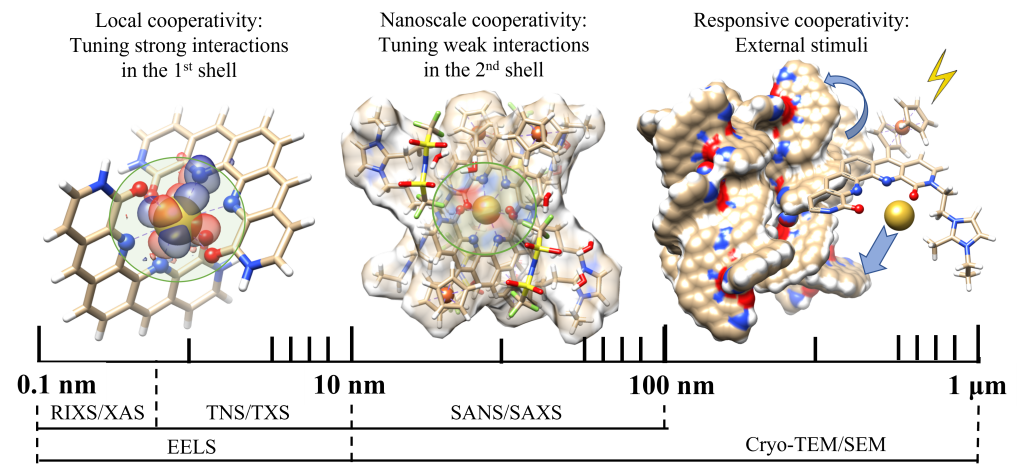
The overarching goal of this research is to develop a fundamental understanding of the synergy between strong interactions at the ligand-metal binding site and weak interactions in the surrounding coordination sphere for the selective separations and stimuli-responsive release of lanthanides. To achieve this goal, the research is organized around two specific aims. In Aim 1, the focus is on understanding the binding principles of lanthanides in the first coordination sphere to design stimuli-responsive, multidentate, preorganized ligands for lanthanide separations. In Aim 2, the focus is on understanding how the solvation environment can be used to control lanthanide selectivity and release. A multimodal approach that combines state-of-the-art experimental characterization methods with computation and machine-learning is being used to elucidate how the molecular structure and solvation environment of the lanthanide complexes correlate them to ion selectivity. This research is taking advantage of recent advances in Cryo-electron microscopy, neutron and X-ray scattering, and spectroscopy methods, along with powerful machine learning algorithms to gain fundamental insights into the interplay between electronic structures of Ln(III) ions and ligands in the immediate coordination environment and higher level superstructures and their effect on selectivity, binding strength, and release mechanisms.







