Filter News
Area of Research
- (-) National Security (24)
- (-) Neutron Science (125)
- (-) Nuclear Science and Technology (39)
- Advanced Manufacturing (4)
- Biology and Environment (59)
- Biology and Soft Matter (1)
- Computational Biology (1)
- Computational Engineering (2)
- Computer Science (9)
- Electricity and Smart Grid (3)
- Energy Frontier Research Centers (1)
- Energy Science (84)
- Fuel Cycle Science and Technology (1)
- Functional Materials for Energy (1)
- Fusion and Fission (29)
- Fusion Energy (10)
- Isotope Development and Production (1)
- Isotopes (4)
- Materials (111)
- Materials for Computing (13)
- Mathematics (1)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Quantum information Science (9)
- Sensors and Controls (1)
- Supercomputing (87)
News Topics
- (-) Big Data (8)
- (-) Grid (6)
- (-) Mathematics (1)
- (-) Nanotechnology (11)
- (-) Neutron Science (122)
- (-) Nuclear Energy (41)
- (-) Physics (11)
- (-) Quantum Science (8)
- 3-D Printing/Advanced Manufacturing (12)
- Advanced Reactors (12)
- Artificial Intelligence (18)
- Bioenergy (11)
- Biology (11)
- Biomedical (18)
- Biotechnology (2)
- Buildings (1)
- Chemical Sciences (5)
- Clean Water (2)
- Composites (1)
- Computer Science (33)
- Coronavirus (13)
- Cybersecurity (19)
- Energy Storage (8)
- Environment (13)
- Exascale Computing (1)
- Fossil Energy (1)
- Frontier (2)
- Fusion (10)
- High-Performance Computing (6)
- Hydropower (1)
- Isotopes (5)
- Machine Learning (15)
- Materials (16)
- Materials Science (27)
- Microscopy (3)
- Molten Salt (4)
- National Security (35)
- Partnerships (5)
- Polymers (1)
- Quantum Computing (1)
- Security (12)
- Simulation (1)
- Space Exploration (8)
- Summit (7)
- Transportation (7)
Media Contacts
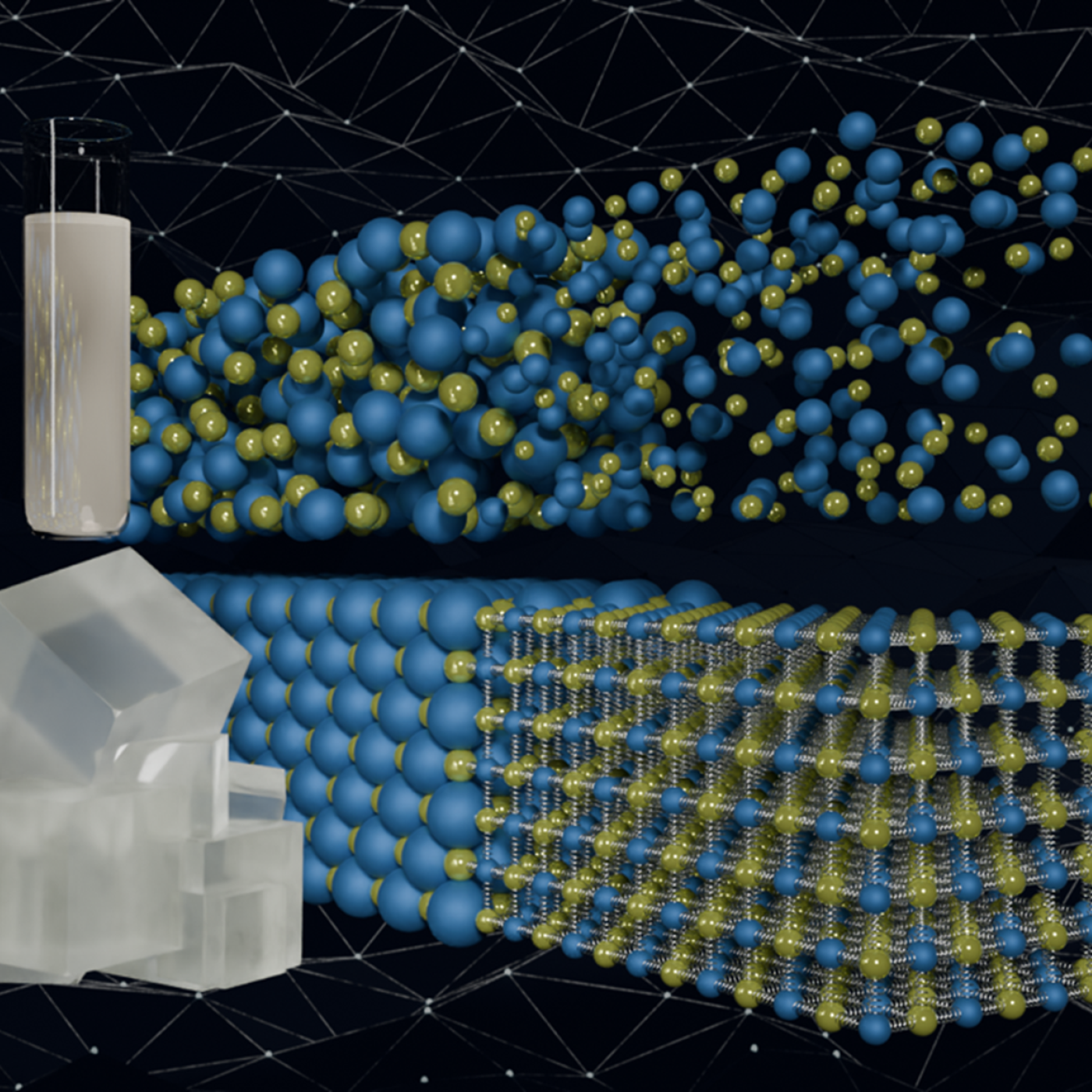
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials

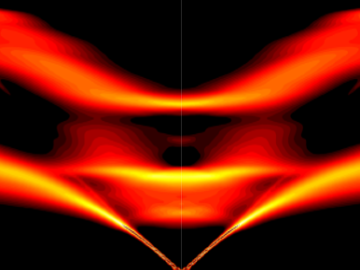
An ORNL-led team's observation of certain crystalline ice phases challenges accepted theories about super-cooled water and non-crystalline ice. Their findings, reported in the journal Nature, will also lead to better understanding of ice and its various phases found on other planets, moons and elsewhere in space.

Tempering, the heating process that gives chocolate its appealing sheen and creamy texture, is a crucial part of crafting quality chocolate. But, at the molecular level, it gets a little tricky, and when done incorrectly, can render entire batches of chocolate gritty and unappetizing.
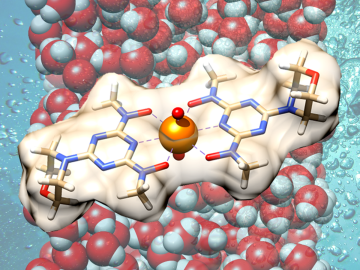
Scientists have demonstrated a new bio-inspired material for an eco-friendly and cost-effective approach to recovering uranium from seawater.

OAK RIDGE, Tenn., May 14, 2019—Advanced Research Systems, Inc., has licensed a technology designed to automatically refill liquid helium used in laboratory equipment for low-temperature scientific experiments, which will reduce downtime, recover more helium and increase overall efficiency.

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
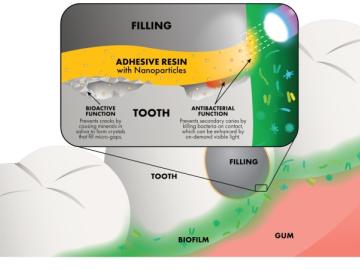
To help address the issue of dental restoration, Oak Ridge National Laboratory researchers are using neutron scattering to study how nanoparticles with antibacterial properties can be added to adhesive resins, which are used by dentists to strengthen the bond between a tooth and its polymer composite filling.

Oak Ridge National Laboratory scientists are evaluating paths for licensing remotely operated microreactors, which could provide clean energy sources to hard-to-reach communities, such as isolated areas in Alaska.

Scientists at the Department of Energy’s Oak Ridge National Laboratory are working to understand both the complex nature of uranium and the various oxide forms it can take during processing steps that might occur throughout the nuclear fuel cycle.

OAK RIDGE, Tenn., March 20, 2019—Direct observations of the structure and catalytic mechanism of a prototypical kinase enzyme—protein kinase A or PKA—will provide researchers and drug developers with significantly enhanced abilities to understand and treat fatal diseases and neurological disorders such as cancer, diabetes, and cystic fibrosis.


