Filter News
Area of Research
- (-) Neutron Science (125)
- (-) Nuclear Science and Technology (12)
- Advanced Manufacturing (22)
- Biological Systems (2)
- Biology and Environment (138)
- Biology and Soft Matter (1)
- Building Technologies (1)
- Computational Biology (2)
- Computational Engineering (2)
- Computer Science (2)
- Electricity and Smart Grid (1)
- Energy Frontier Research Centers (1)
- Energy Science (211)
- Functional Materials for Energy (1)
- Fusion and Fission (11)
- Fusion Energy (1)
- Isotopes (7)
- Materials (118)
- Materials for Computing (18)
- Mathematics (1)
- National Security (20)
- Quantum information Science (2)
- Supercomputing (88)
- Transportation Systems (2)
News Topics
- (-) 3-D Printing/Advanced Manufacturing (10)
- (-) Bioenergy (8)
- (-) Biomedical (16)
- (-) Environment (13)
- (-) Nanotechnology (10)
- (-) Neutron Science (122)
- (-) Transportation (5)
- Advanced Reactors (11)
- Artificial Intelligence (6)
- Big Data (2)
- Biology (8)
- Biotechnology (1)
- Chemical Sciences (3)
- Clean Water (2)
- Composites (1)
- Computer Science (16)
- Coronavirus (11)
- Cybersecurity (2)
- Energy Storage (6)
- Fossil Energy (1)
- Frontier (1)
- Fusion (9)
- High-Performance Computing (2)
- Hydropower (1)
- Isotopes (5)
- Machine Learning (3)
- Materials (14)
- Materials Science (26)
- Mathematics (1)
- Mercury (1)
- Microscopy (3)
- Molten Salt (4)
- National Security (2)
- Nuclear Energy (38)
- Physics (10)
- Polymers (1)
- Quantum Computing (1)
- Quantum Science (7)
- Security (2)
- Space Exploration (8)
- Summit (6)
Media Contacts
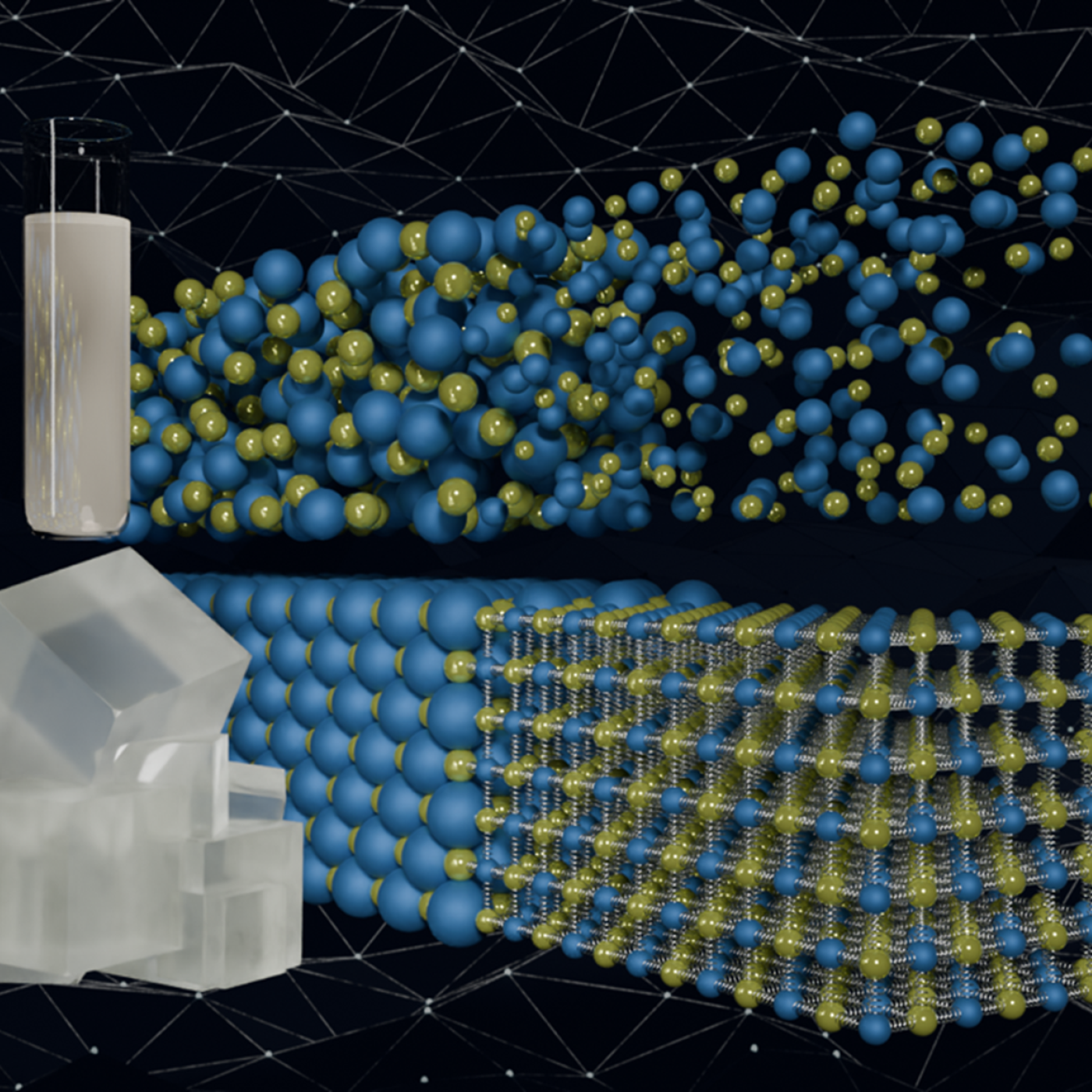
Oak Ridge National Laboratory researchers working on neutron imaging capabilities for nuclear materials have developed a process for seeing the inside of uranium particles – without cutting them open.

A versatile class of flexible, protein-like polymers could significantly advance future drug delivery methods. But first, scientists have to develop a reliable process for tailoring these polymers into shapes that can effectively transport medicines throughout the human body.

Biological membranes, such as the “walls” of most types of living cells, primarily consist of a double layer of lipids, or “lipid bilayer,” that forms the structure, and a variety of embedded and attached proteins with highly specialized functions, including proteins that rapidly and selectively transport ions and molecules in and out of the cell.

OAK RIDGE, Tenn., Feb. 19, 2020 — The U.S. Department of Energy’s Oak Ridge National Laboratory and the Tennessee Valley Authority have signed a memorandum of understanding to evaluate a new generation of flexible, cost-effective advanced nuclear reactors.
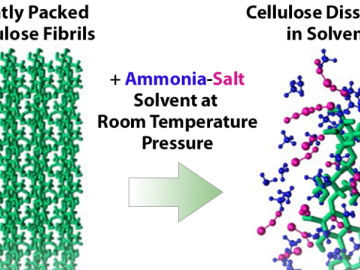
Researchers have developed a new process that could make it much cheaper to produce biofuels such as ethanol from plant waste and reduce reliance on fossil fuels.

Illustration of the optimized zeolite catalyst, or NbAlS-1, which enables a highly efficient chemical reaction to create butene, a renewable source of energy, without expending high amounts of energy for the conversion. Credit: Jill Hemman, Oak Ridge National Laboratory/U.S. Dept. of Energy
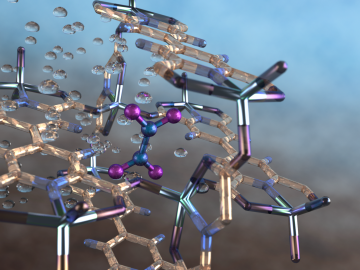
An international team of scientists, led by the University of Manchester, has developed a metal-organic framework, or MOF, material

Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory have new experimental evidence and a predictive theory that solves a long-standing materials science mystery: why certain crystalline materials shrink when heated.

In the vast frozen whiteness of the central Arctic, the Polarstern, a German research vessel, has settled into the ice for a yearlong float.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.


