Filter News
Area of Research
News Topics
- (-) Chemical Sciences (86)
- (-) Neutron Science (171)
- 3-D Printing/Advanced Manufacturing (146)
- Advanced Reactors (40)
- Artificial Intelligence (131)
- Big Data (79)
- Bioenergy (112)
- Biology (128)
- Biomedical (73)
- Biotechnology (39)
- Buildings (74)
- Clean Water (33)
- Composites (35)
- Computer Science (226)
- Coronavirus (48)
- Critical Materials (29)
- Cybersecurity (35)
- Education (5)
- Element Discovery (1)
- Emergency (4)
- Energy Storage (114)
- Environment (218)
- Exascale Computing (67)
- Fossil Energy (8)
- Frontier (64)
- Fusion (66)
- Grid (74)
- High-Performance Computing (130)
- Hydropower (12)
- Irradiation (3)
- Isotopes (62)
- ITER (9)
- Machine Learning (68)
- Materials (157)
- Materials Science (158)
- Mathematics (12)
- Mercury (12)
- Microelectronics (4)
- Microscopy (56)
- Molten Salt (10)
- Nanotechnology (64)
- National Security (86)
- Nuclear Energy (122)
- Partnerships (68)
- Physics (69)
- Polymers (35)
- Quantum Computing (53)
- Quantum Science (93)
- Security (31)
- Simulation (65)
- Software (1)
- Space Exploration (26)
- Statistics (4)
- Summit (71)
- Transportation (103)
Media Contacts
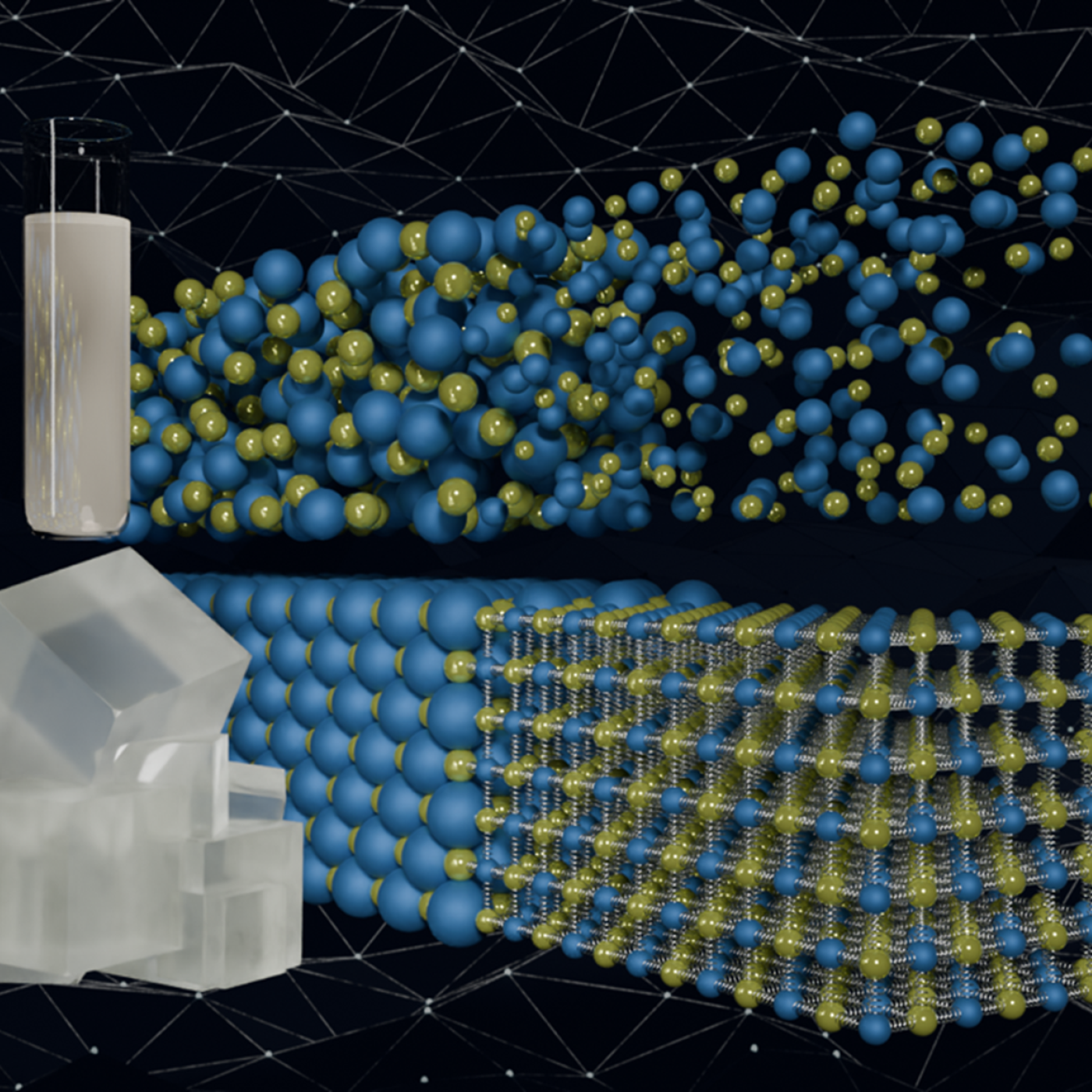
Scientists have developed a new machine learning approach that accurately predicted critical and difficult-to-compute properties of molten salts, materials with diverse nuclear energy applications.
Using the now-decommissioned Summit supercomputer, researchers at ORNL ran the largest and most accurate molecular dynamics simulations yet of the interface between water and air during a chemical reaction. The simulations have uncovered how water controls such chemical reactions by dynamically coupling with the molecules involved in the process.

Van Graves, an engineering manager at ORNL, is celebrating 40 years of dedicated service leading a diverse range of prominent engineering projects at ORNL and internationally.

Using the Frontier supercomputer, a team of researchers from the Massachusetts Institute of Technology conducted large-scale calculations to chart the isospin density of a neutron star across a range of conditions. Their work provides new insights into how pressure and density interact within neutron stars, offering important predictions about their inner workings.
Scientists at ORNL and the University of Cincinnati achieved a breakthrough in understanding the vulnerability of microbes to the butanol they produce during fermentation of plant biomass. The discovery could pave the way for more efficient production of domestic fuels, chemicals and materials.
To help reduce the likelihood of losing future cultivated crops to drought and other seasonal hardships, researchers from ORNL, Budapest and Hungary are using neutrons, light microscopy and transmission electron microscopy to study the 'Never Never' plant, known for its ability to endure periods of little to no rain.

During his first visit to Oak Ridge National Laboratory, Energy Secretary Chris Wright compared the urgency of the Lab’s World War II beginnings to today’s global race to lead in artificial intelligence, calling for a “Manhattan Project 2.”

A workshop led by scientists at ORNL sketched a road map toward a longtime goal: development of autonomous, or self-driving, next-generation research laboratories.

Hugh O’Neill’s lifelong fascination with the complexities of the natural world drives his research at ORNL, where he’s using powerful neutron beams to dive deep into the microscopic realm of biological materials and unlock secrets for better production of domestic biofuels and bioproducts.

A team of scientists led by a professor from Duke University discovered a way to help make batteries safer, charge faster and last longer. They relied on neutrons at ORNL to understand at the atomic scale how lithium moves in lithium phosphorus sulfur chloride, a promising new type of solid-state battery material known as a superionic compound.


