Project Details
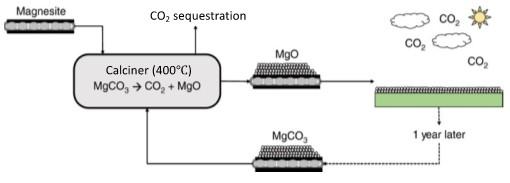
The overarching goal of this project is to understand and control the chemical reactions that govern hydroxylation and carbonation processes during Direct Air Capture (DAC) of CO2 using magnesium oxide (MgO). This goal will be achieved by resolving three gaps in our fundamental understanding of the reactions of CO2 with MgO that inhibit our ability to capitalize on this promising method to capture dilute CO2 on the gigaton scale. This proposal is divided into three Specific Aims focused on the major knowledge gaps: Specific Aim #1 What are the chemical reactions that control the rate and extent of reaction of MgO with CO2 at ambient conditions and varying humidity? In addition to uncertainties about the rates of carbonation under ambient conditions, another potential challenge for MgO looping is the formation of passivating layers consisting of hydrated carbonate phases which limit the reaction with CO2. Thus, in SA1 we will focus on identifying the factors that control phase selection and the kinetics of carbonation under ambient conditions. By understanding these factors, we will be able to predict and control the rate and extent of the reactions. Specific Aim #2) What are the mechanisms of reaction-induced fracturing during MgO hydroxylation? A second challenge in the application of mineral looping methods for CO2 capture is sintering during calcining, which releases CO2 and regenerates the MgO. This leads to detrimental reductions in surface area and thus CO2 sorption capacity. However, engineered reaction-induced fracturing may allow us to increase the surface area after calcining steps, extending the lifetime of the sorbent, and enhancing the economics. However, the reactive processes by which this process occurs are not understood, inhibiting our ability to use this phenomenon beneficially. Specific Aim #3) What are the roles of impurities in affecting the hydroxylation, carbonation, and oxide regeneration reactions? Impurities can be either present in the MgO solid itself (either as a dopant or a segregated secondary phase) or as aqueous solutes at the solid-water interface. We hypothesize that it may be possible to control phase selection during carbonation beneficially by selective impurity incorporation. To address these three questions, we will combine kinetic and mechanistic investigations with advanced X-ray/neutron scattering and chemical imaging with atomic to meso-scale phase field modeling approaches. By achieving this goal, we will provide the scientific foundation to enable direct air capture of CO2 on the gigaton scale. With the proposed research we will meet critical needs described in the Carbon Negative Earthshot and the BES roundtable “Foundational Science for Carbon Dioxide Removal Technologies”, specifically, the Priority Research Opportunities (PRO) PRO1 “Master Interfacial Processes of CO2 Transport and Reactivity Across Multiple Length and Time Scales” and PRO4 “Control Multiphase Interactions Required for CO2 Conversion into Molecules, Minerals and Materials”.





