Filter News
Area of Research
News Topics
- (-) Isotopes (6)
- (-) Polymers (7)
- 3-D Printing/Advanced Manufacturing (6)
- Advanced Reactors (1)
- Artificial Intelligence (2)
- Big Data (2)
- Bioenergy (4)
- Biomedical (4)
- Chemical Sciences (2)
- Climate Change (1)
- Composites (2)
- Computer Science (9)
- Coronavirus (1)
- Critical Materials (2)
- Cybersecurity (1)
- Energy Storage (9)
- Environment (3)
- Exascale Computing (1)
- Fusion (1)
- Grid (1)
- Machine Learning (3)
- Materials (1)
- Materials Science (37)
- Mathematics (1)
- Microscopy (9)
- Molten Salt (1)
- Nanotechnology (18)
- National Security (1)
- Neutron Science (13)
- Nuclear Energy (5)
- Physics (12)
- Quantum Science (4)
- Security (1)
- Space Exploration (1)
- Summit (2)
- Sustainable Energy (5)
- Transformational Challenge Reactor (2)
- Transportation (4)
Media Contacts

Six scientists at the Department of Energy’s Oak Ridge National Laboratory were named Battelle Distinguished Inventors, in recognition of obtaining 14 or more patents during their careers at the lab.
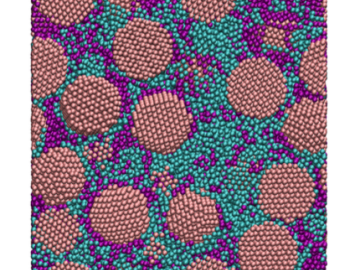
Oak Ridge National Laboratory scientists have discovered a cost-effective way to significantly improve the mechanical performance of common polymer nanocomposite materials.
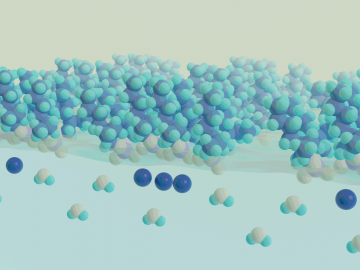
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.
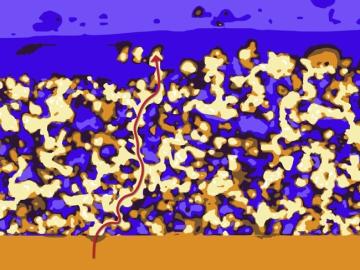
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
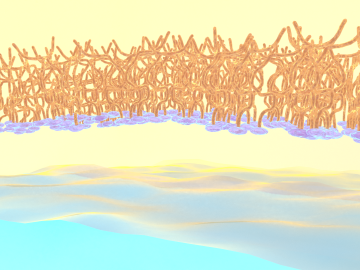
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.

Carbon fiber composites—lightweight and strong—are great structural materials for automobiles, aircraft and other transportation vehicles. They consist of a polymer matrix, such as epoxy, into which reinforcing carbon fibers have been embedded. Because of differences in the mecha...
Physicists turned to the “doubly magic” tin isotope Sn-132, colliding it with a target at Oak Ridge National Laboratory to assess its properties as it lost a neutron to become Sn-131.
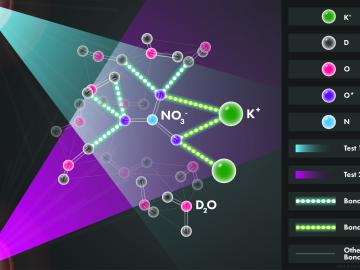
Scientists at the Department of Energy’s Oak Ridge National Laboratory used neutrons, isotopes and simulations to “see” the atomic structure of a saturated solution and found evidence supporting one of two competing hypotheses about how ions come

Oak Ridge National Laboratory scientists have developed a crucial component for a new kind of low-cost stationary battery system utilizing common materials and designed for grid-scale electricity storage. Large, economical electricity storage systems can benefit the nation’s grid ...
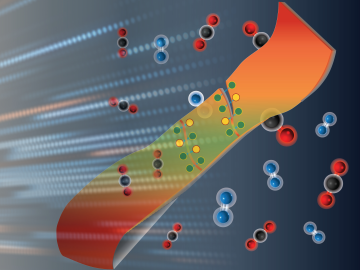
An Oak Ridge National Laboratory–led team has developed super-stretchy polymers with amazing self-healing abilities that could lead to longer-lasting consumer products.




