Filter News
Area of Research
News Topics
- (-) Energy Storage (14)
- 3-D Printing/Advanced Manufacturing (22)
- Advanced Reactors (9)
- Artificial Intelligence (27)
- Big Data (12)
- Bioenergy (16)
- Biology (9)
- Biomedical (10)
- Biotechnology (5)
- Buildings (10)
- Chemical Sciences (8)
- Clean Water (8)
- Climate Change (14)
- Composites (5)
- Computer Science (48)
- Critical Materials (2)
- Cybersecurity (5)
- Decarbonization (14)
- Emergency (1)
- Environment (35)
- Exascale Computing (4)
- Fossil Energy (2)
- Frontier (5)
- Fusion (9)
- Grid (8)
- High-Performance Computing (11)
- Isotopes (6)
- ITER (1)
- Machine Learning (10)
- Materials (10)
- Materials Science (28)
- Mathematics (1)
- Mercury (1)
- Microscopy (6)
- Molten Salt (1)
- Nanotechnology (8)
- National Security (10)
- Net Zero (3)
- Neutron Science (26)
- Nuclear Energy (23)
- Partnerships (8)
- Physics (7)
- Polymers (5)
- Quantum Computing (5)
- Quantum Science (18)
- Security (4)
- Simulation (8)
- Space Exploration (6)
- Statistics (2)
- Summit (13)
- Sustainable Energy (18)
- Transportation (20)
Media Contacts

An international team using neutrons set the first benchmark (one nanosecond) for a polymer-electrolyte and lithium-salt mixture. Findings could produce safer, more powerful lithium batteries.

Shift Thermal, a member of Innovation Crossroads’ first cohort of fellows, is commercializing advanced ice thermal energy storage for HVAC, shifting the cooling process to be more sustainable, cost-effective and resilient. Shift Thermal wants to enable a lower-cost, more-efficient thermal energy storage method to provide long-duration resilient cooling when the electric grid is down.

The Department of Energy’s Oak Ridge National Laboratory is providing national leadership in a new collaboration among five national laboratories to accelerate U.S. production of clean hydrogen fuel cells and electrolyzers.
Chelsea Chen, a polymer physicist at ORNL, is studying ion transport in solid electrolytes that could help electric vehicle battery charges last longer.

Technology Transfer staff from Department of Energy’s Oak Ridge National Laboratory attended the 2024 Consumer Electronics Show, or CES, in Las Vegas, Jan. 8–12.

Electric vehicles can drive longer distances if their lithium-ion batteries deliver more energy in a lighter package. A prime weight-loss candidate is the current collector, a component that often adds 10% to the weight of a battery cell without contributing energy.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.
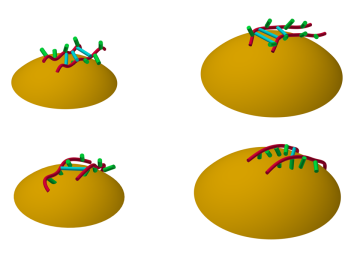
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.
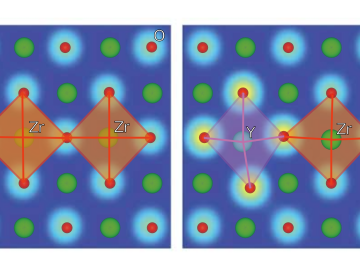
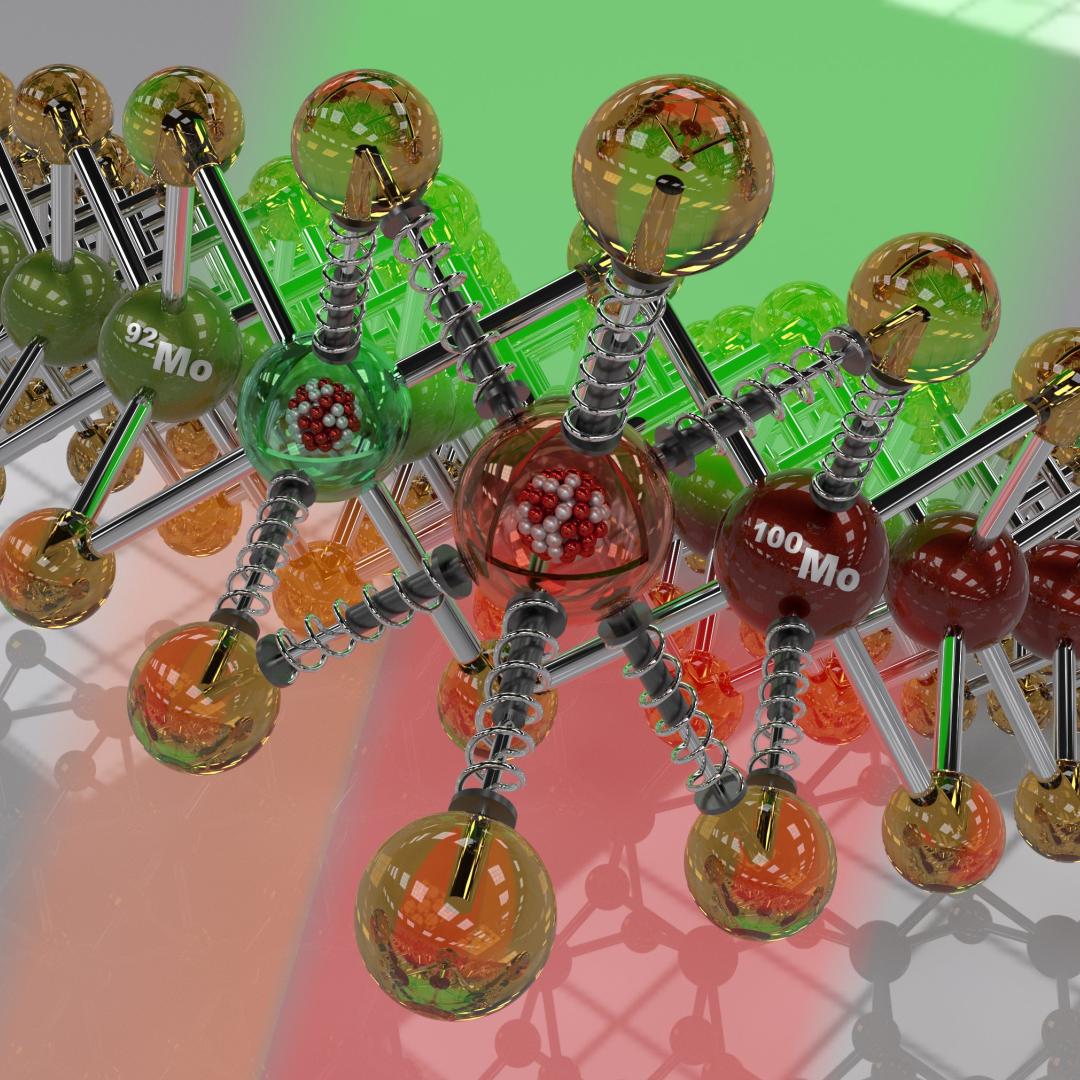
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.