Filter News
Area of Research
News Topics
- (-) Clean Water (5)
- (-) Energy Storage (8)
- 3-D Printing/Advanced Manufacturing (15)
- Advanced Reactors (7)
- Artificial Intelligence (12)
- Big Data (7)
- Bioenergy (9)
- Biomedical (5)
- Biotechnology (1)
- Composites (2)
- Computer Science (35)
- Cybersecurity (5)
- Environment (19)
- Exascale Computing (2)
- Frontier (2)
- Fusion (5)
- Grid (5)
- Isotopes (1)
- Machine Learning (5)
- Materials Science (20)
- Mercury (1)
- Microscopy (5)
- Molten Salt (1)
- Nanotechnology (6)
- Neutron Science (18)
- Nuclear Energy (17)
- Physics (6)
- Polymers (2)
- Quantum Science (10)
- Security (2)
- Space Exploration (4)
- Summit (9)
- Sustainable Energy (8)
- Transportation (12)
Media Contacts

While Tsouris’ water research is diverse in scope, its fundamentals are based on basic science principles that remain largely unchanged, particularly in a mature field like chemical engineering.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.

The National Alliance for Water Innovation, a partnership of the Department of Energy’s Oak Ridge National Laboratory, other national labs, university and private sector partners, has been awarded a five-year, $100 million Energy-Water Desalination Hub by DOE to address water security issues in the United States.
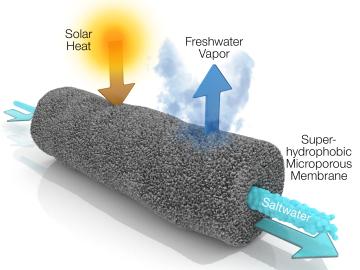
A new method developed at Oak Ridge National Laboratory improves the energy efficiency of a desalination process known as solar-thermal evaporation.
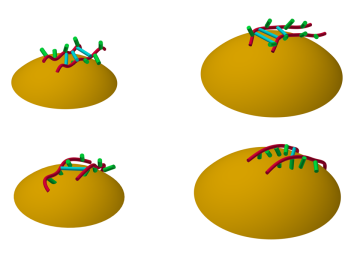
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
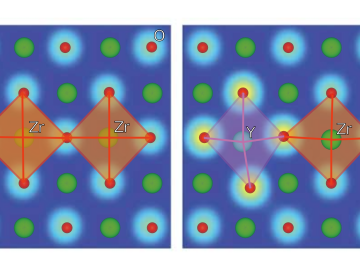
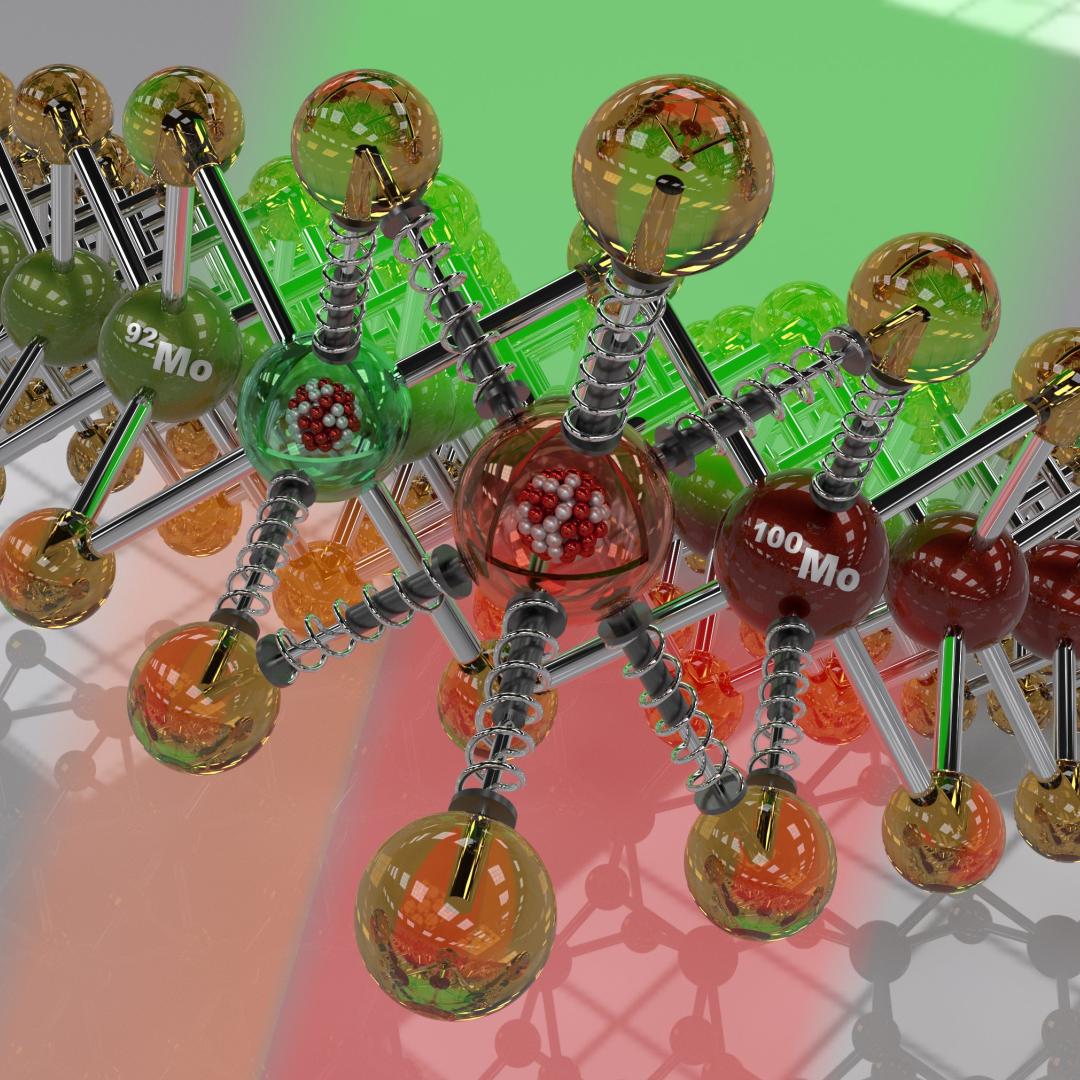
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
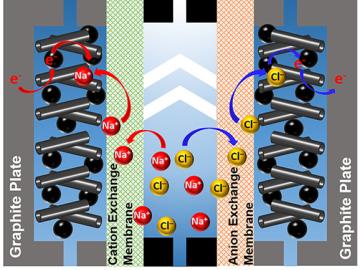
A team of scientists led by Oak Ridge National Laboratory used carbon nanotubes to improve a desalination process that attracts and removes ionic compounds such as salt from water using charged electrodes.

The use of lithium-ion batteries has surged in recent years, starting with electronics and expanding into many applications, including the growing electric and hybrid vehicle industry. But the technologies to optimize recycling of these batteries have not kept pace.