
Filter News
Area of Research
- Advanced Manufacturing (4)
- Biological Systems (1)
- Biology and Environment (2)
- Clean Energy (28)
- Climate and Environmental Systems (2)
- Computer Science (2)
- Fusion Energy (2)
- Materials (27)
- National Security (2)
- Neutron Science (7)
- Nuclear Science and Technology (2)
- Quantum information Science (2)
- Supercomputing (9)
- Transportation Systems (1)
News Topics
- (-) Bioenergy (9)
- (-) Clean Water (5)
- (-) Cybersecurity (5)
- (-) Environment (19)
- (-) Grid (5)
- (-) Isotopes (1)
- (-) Materials Science (20)
- (-) Microscopy (5)
- (-) Physics (6)
- (-) Space Exploration (4)
- 3-D Printing/Advanced Manufacturing (15)
- Advanced Reactors (7)
- Artificial Intelligence (12)
- Big Data (7)
- Biomedical (5)
- Biotechnology (1)
- Composites (2)
- Computer Science (35)
- Energy Storage (8)
- Exascale Computing (2)
- Frontier (2)
- Fusion (5)
- Machine Learning (5)
- Mercury (1)
- Molten Salt (1)
- Nanotechnology (6)
- Neutron Science (18)
- Nuclear Energy (17)
- Polymers (2)
- Quantum Science (10)
- Security (2)
- Summit (9)
- Sustainable Energy (8)
- Transportation (12)
Media Contacts
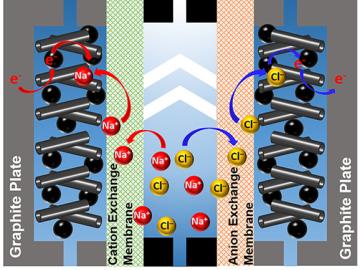
A team of scientists led by Oak Ridge National Laboratory used carbon nanotubes to improve a desalination process that attracts and removes ionic compounds such as salt from water using charged electrodes.

Kevin Field at the Department of Energy’s Oak Ridge National Laboratory synthesizes and scrutinizes materials for nuclear power systems that must perform safely and efficiently over decades of irradiation.

OAK RIDGE, Tenn., March 22, 2019 – Karren Leslie More, a researcher at the Department of Energy’s Oak Ridge National Laboratory, has been elected fellow of the Microscopy Society of America (MSA) professional organization.

OAK RIDGE, Tenn., March 11, 2019—An international collaboration including scientists at the Department of Energy’s Oak Ridge National Laboratory solved a 50-year-old puzzle that explains why beta decays of atomic nuclei
Higher carbon dioxide levels caused 30 percent more wood growth in young forest stands across the temperate United States over a decade, according to an analysis led by Oak Ridge National Laboratory.
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

The use of lithium-ion batteries has surged in recent years, starting with electronics and expanding into many applications, including the growing electric and hybrid vehicle industry. But the technologies to optimize recycling of these batteries have not kept pace.

More than 1800 years ago, Chinese astronomers puzzled over the sudden appearance of a bright “guest star” in the sky, unaware that they were witnessing the cosmic forge of a supernova, an event repeated countless times scattered across the universe.


