
Filter News
Area of Research
- Advanced Manufacturing (1)
- Biology and Environment (26)
- Clean Energy (38)
- Climate and Environmental Systems (2)
- Computational Biology (1)
- Computational Engineering (2)
- Computer Science (5)
- Fusion and Fission (1)
- Isotopes (2)
- Materials (11)
- Materials for Computing (5)
- Mathematics (1)
- National Security (1)
- Neutron Science (10)
- Nuclear Science and Technology (2)
- Supercomputing (16)
News Type
News Topics
- (-) Artificial Intelligence (14)
- (-) Biotechnology (5)
- (-) Energy Storage (20)
- (-) Environment (51)
- (-) Microscopy (9)
- (-) Polymers (4)
- (-) Security (2)
- (-) Space Exploration (8)
- 3-D Printing/Advanced Manufacturing (30)
- Advanced Reactors (8)
- Big Data (11)
- Bioenergy (16)
- Biology (18)
- Biomedical (9)
- Buildings (9)
- Chemical Sciences (3)
- Clean Water (12)
- Climate Change (10)
- Composites (4)
- Computer Science (48)
- Coronavirus (6)
- Critical Materials (2)
- Cybersecurity (3)
- Decarbonization (6)
- Exascale Computing (3)
- Frontier (1)
- Fusion (6)
- Grid (12)
- High-Performance Computing (14)
- Isotopes (8)
- ITER (3)
- Machine Learning (4)
- Materials (21)
- Materials Science (27)
- Mathematics (1)
- Mercury (4)
- Molten Salt (1)
- Nanotechnology (9)
- National Security (4)
- Net Zero (1)
- Neutron Science (23)
- Nuclear Energy (20)
- Physics (6)
- Quantum Computing (2)
- Quantum Science (12)
- Statistics (1)
- Summit (10)
- Sustainable Energy (32)
- Transportation (27)
Media Contacts
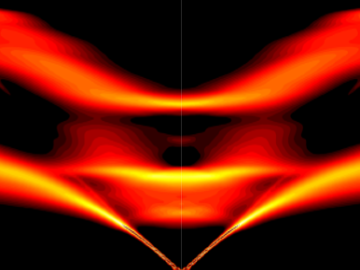
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
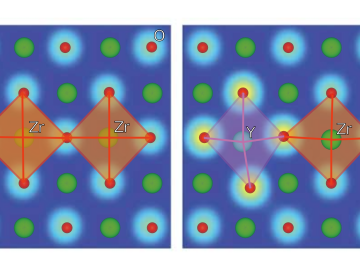
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
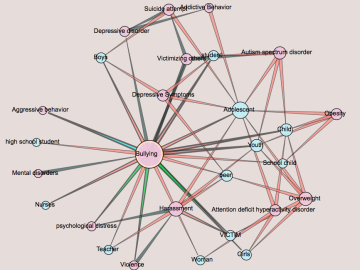
Oak Ridge National Laboratory is using artificial intelligence to analyze data from published medical studies associated with bullying to reveal the potential of broader impacts, such as mental illness or disease.
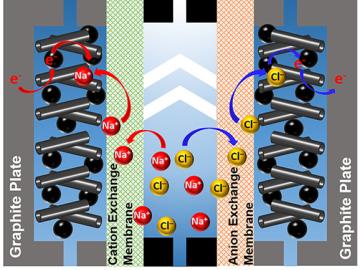
A team of scientists led by Oak Ridge National Laboratory used carbon nanotubes to improve a desalination process that attracts and removes ionic compounds such as salt from water using charged electrodes.
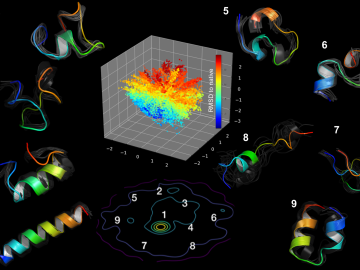
Using artificial neural networks designed to emulate the inner workings of the human brain, deep-learning algorithms deftly peruse and analyze large quantities of data. Applying this technique to science problems can help unearth historically elusive solutions.
Higher carbon dioxide levels caused 30 percent more wood growth in young forest stands across the temperate United States over a decade, according to an analysis led by Oak Ridge National Laboratory.

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

The use of lithium-ion batteries has surged in recent years, starting with electronics and expanding into many applications, including the growing electric and hybrid vehicle industry. But the technologies to optimize recycling of these batteries have not kept pace.
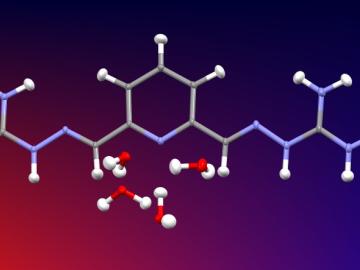
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.


