Filter News
Area of Research
News Topics
- (-) 3-D Printing/Advanced Manufacturing (3)
- (-) Energy Storage (10)
- Advanced Reactors (6)
- Artificial Intelligence (3)
- Big Data (5)
- Biology (2)
- Biomedical (6)
- Buildings (2)
- Chemical Sciences (1)
- Clean Water (2)
- Climate Change (9)
- Composites (1)
- Computer Science (7)
- Coronavirus (3)
- Critical Materials (1)
- Decarbonization (2)
- Environment (11)
- Frontier (1)
- Fusion (3)
- Grid (3)
- High-Performance Computing (2)
- Isotopes (1)
- ITER (1)
- Machine Learning (5)
- Materials (2)
- Materials Science (10)
- Microscopy (2)
- Molten Salt (1)
- Nanotechnology (2)
- Neutron Science (5)
- Nuclear Energy (6)
- Polymers (3)
- Simulation (2)
- Summit (3)
- Sustainable Energy (10)
- Transportation (6)
Media Contacts
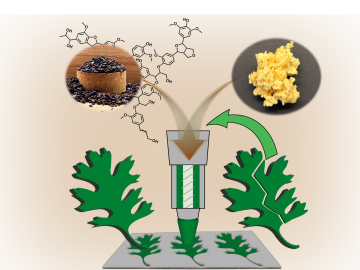
Oak Ridge National Laboratory scientists ingeniously created a sustainable, soft material by combining rubber with woody reinforcements and incorporating “smart” linkages between the components that unlock on demand.
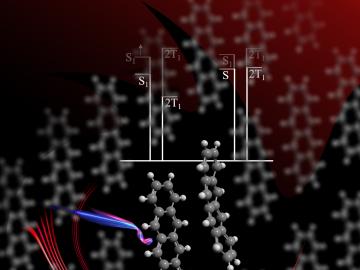
ORNL scientists develop a sample holder that tumbles powdered photochemical materials within a neutron beamline — exposing more of the material to light for increased photo-activation and better photochemistry data capture.

An international team using neutrons set the first benchmark (one nanosecond) for a polymer-electrolyte and lithium-salt mixture. Findings could produce safer, more powerful lithium batteries.

Electric vehicles can drive longer distances if their lithium-ion batteries deliver more energy in a lighter package. A prime weight-loss candidate is the current collector, a component that often adds 10% to the weight of a battery cell without contributing energy.
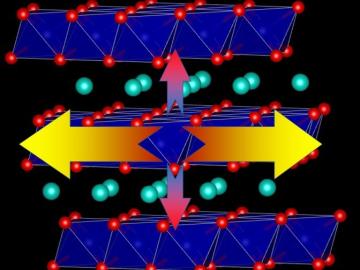
Oak Ridge National Laboratory researchers proved that the heat transport ability of lithium-ion battery cathodes is much lower than previously determined, a finding that could help explain barriers to increasing energy storage capacity and boosting performance.
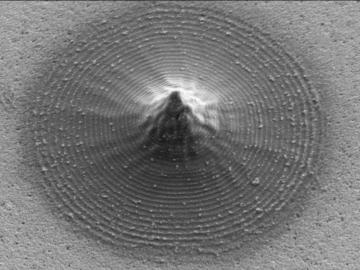
Scientists at Oak Ridge National Laboratory and the University of Tennessee designed and demonstrated a method to make carbon-based materials that can be used as electrodes compatible with a specific semiconductor circuitry.
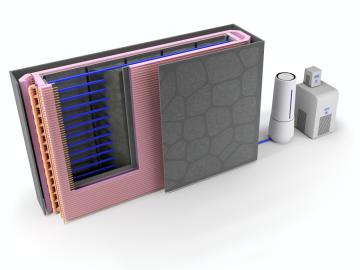
Oak Ridge National Laboratory researchers used additive manufacturing to build a first-of-its kind smart wall called EMPOWER.
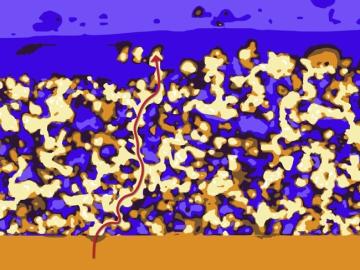
Oak Ridge National Laboratory scientists seeking the source of charge loss in lithium-ion batteries demonstrated that coupling a thin-film cathode with a solid electrolyte is a rapid way to determine the root cause.
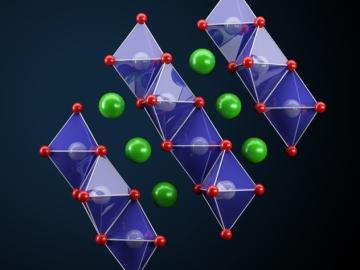
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.

Researchers at Oak Ridge National Laboratory demonstrated a 20-kilowatt bi-directional wireless charging system on a UPS plug-in hybrid electric delivery truck, advancing the technology to a larger class of vehicles and enabling a new energy storage method for fleet owners and their facilities.