Filter News
Area of Research
News Type
News Topics
- (-) Advanced Reactors (1)
- (-) Polymers (4)
- 3-D Printing/Advanced Manufacturing (4)
- Artificial Intelligence (2)
- Big Data (2)
- Bioenergy (3)
- Biomedical (1)
- Chemical Sciences (2)
- Climate Change (1)
- Computer Science (8)
- Coronavirus (1)
- Critical Materials (2)
- Cybersecurity (1)
- Energy Storage (8)
- Environment (3)
- Exascale Computing (1)
- Isotopes (1)
- Machine Learning (3)
- Materials (1)
- Materials Science (30)
- Mathematics (1)
- Microscopy (5)
- Molten Salt (1)
- Nanotechnology (12)
- National Security (1)
- Neutron Science (10)
- Nuclear Energy (3)
- Physics (8)
- Quantum Science (4)
- Security (1)
- Summit (2)
- Sustainable Energy (5)
- Transformational Challenge Reactor (2)
- Transportation (3)
Media Contacts
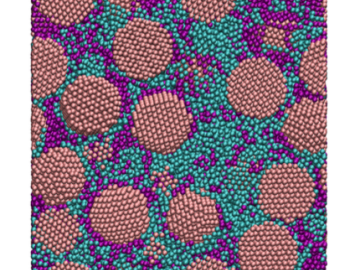
Oak Ridge National Laboratory scientists have discovered a cost-effective way to significantly improve the mechanical performance of common polymer nanocomposite materials.
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.

Scientists at the Department of Energy Manufacturing Demonstration Facility at ORNL have their eyes on the prize: the Transformational Challenge Reactor, or TCR, a microreactor built using 3D printing and other new approaches that will be up and running by 2023.
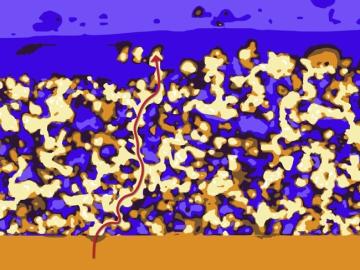
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
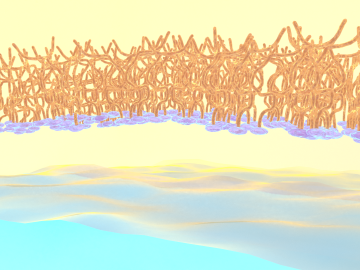
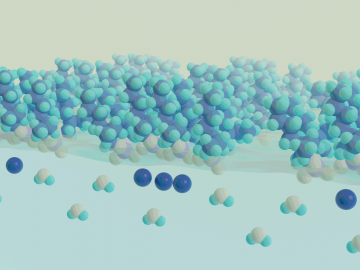
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.