Filter News
Area of Research
- (-) Materials (72)
- Advanced Manufacturing (2)
- Biology and Environment (83)
- Biology and Soft Matter (1)
- Clean Energy (59)
- Climate and Environmental Systems (2)
- Computational Biology (1)
- Computational Engineering (1)
- Computer Science (5)
- Fusion and Fission (12)
- Fusion Energy (1)
- Isotope Development and Production (1)
- Isotopes (3)
- Materials Characterization (1)
- Materials for Computing (11)
- Materials Under Extremes (1)
- National Security (22)
- Neutron Science (31)
- Nuclear Science and Technology (9)
- Quantum information Science (2)
- Supercomputing (94)
News Type
News Topics
- (-) Advanced Reactors (2)
- (-) Computer Science (16)
- (-) Environment (13)
- (-) Exascale Computing (2)
- (-) Materials Science (52)
- 3-D Printing/Advanced Manufacturing (17)
- Artificial Intelligence (8)
- Big Data (2)
- Bioenergy (10)
- Biology (4)
- Biomedical (5)
- Buildings (3)
- Chemical Sciences (27)
- Clean Water (2)
- Climate Change (5)
- Composites (5)
- Coronavirus (3)
- Critical Materials (8)
- Cybersecurity (4)
- Decarbonization (5)
- Energy Storage (25)
- Frontier (2)
- Fusion (4)
- Grid (4)
- High-Performance Computing (3)
- Isotopes (11)
- ITER (1)
- Machine Learning (4)
- Materials (57)
- Mathematics (1)
- Microscopy (18)
- Molten Salt (2)
- Nanotechnology (29)
- National Security (3)
- Net Zero (1)
- Neutron Science (27)
- Nuclear Energy (11)
- Partnerships (11)
- Physics (25)
- Polymers (10)
- Quantum Computing (2)
- Quantum Science (10)
- Renewable Energy (1)
- Security (2)
- Space Exploration (1)
- Summit (2)
- Sustainable Energy (9)
- Transformational Challenge Reactor (3)
- Transportation (8)
Media Contacts

OAK RIDGE, Tenn., May 7, 2019—Energy Secretary Rick Perry, Congressman Chuck Fleischmann and lab officials today broke ground on a multipurpose research facility that will provide state-of-the-art laboratory space

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
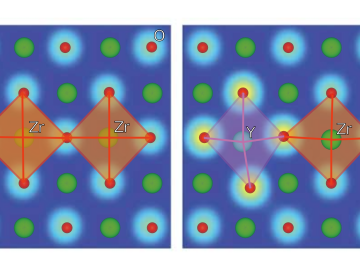
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and

OAK RIDGE, Tenn., Feb. 8, 2019—The Department of Energy’s Oak Ridge National Laboratory has named Sean Hearne director of the Center for Nanophase Materials Sciences. The center is a DOE Office of Science User Facility that brings world-leading resources and capabilities to the nanoscience resear...
Scientists at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have developed a process that could remove CO2 from coal-burning power plant emissions in a way that is similar to how soda lime works in scuba diving rebreathers. Their research, published January 31 in...

Carbon fiber composites—lightweight and strong—are great structural materials for automobiles, aircraft and other transportation vehicles. They consist of a polymer matrix, such as epoxy, into which reinforcing carbon fibers have been embedded. Because of differences in the mecha...
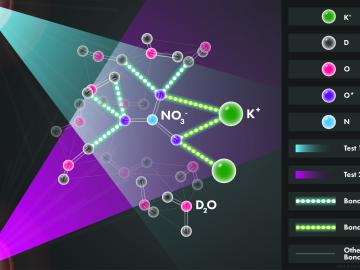
Scientists at the Department of Energy’s Oak Ridge National Laboratory used neutrons, isotopes and simulations to “see” the atomic structure of a saturated solution and found evidence supporting one of two competing hypotheses about how ions come

A tiny vial of gray powder produced at the Department of Energy’s Oak Ridge National Laboratory is the backbone of a new experiment to study the intense magnetic fields created in nuclear collisions.


