
Filter News
Area of Research
- (-) Materials (35)
- Advanced Manufacturing (1)
- Biology and Environment (10)
- Clean Energy (45)
- Computer Science (1)
- Energy Sciences (1)
- Fusion and Fission (7)
- Fusion Energy (6)
- Isotopes (19)
- Materials for Computing (2)
- National Security (1)
- Neutron Science (6)
- Nuclear Science and Technology (15)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Quantum information Science (1)
- Supercomputing (6)
News Type
News Topics
- (-) Advanced Reactors (2)
- (-) Energy Storage (13)
- (-) Isotopes (8)
- (-) Molten Salt (1)
- (-) Physics (13)
- 3-D Printing/Advanced Manufacturing (10)
- Artificial Intelligence (4)
- Big Data (2)
- Bioenergy (3)
- Biomedical (4)
- Buildings (2)
- Chemical Sciences (11)
- Clean Water (3)
- Composites (6)
- Computer Science (9)
- Coronavirus (2)
- Critical Materials (5)
- Cybersecurity (1)
- Decarbonization (2)
- Environment (7)
- Exascale Computing (1)
- Fusion (4)
- Grid (2)
- High-Performance Computing (1)
- Machine Learning (2)
- Materials (31)
- Materials Science (36)
- Mathematics (1)
- Microscopy (12)
- Nanotechnology (16)
- Neutron Science (13)
- Nuclear Energy (12)
- Partnerships (3)
- Polymers (10)
- Quantum Computing (2)
- Quantum Science (1)
- Security (1)
- Space Exploration (2)
- Summit (1)
- Sustainable Energy (5)
- Transformational Challenge Reactor (2)
- Transportation (10)
Media Contacts
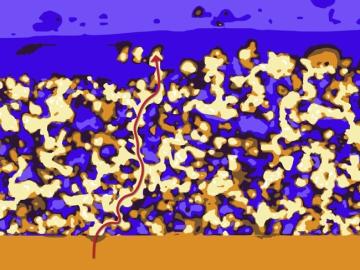
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.

In the Physics Division of the Department of Energy’s Oak Ridge National Laboratory, James (“Mitch”) Allmond conducts experiments and uses theoretical models to advance our understanding of the structure of atomic nuclei, which are made of various combinations of protons and neutrons (nucleons).

The formation of lithium dendrites is still a mystery, but materials engineers study the conditions that enable dendrites and how to stop them.
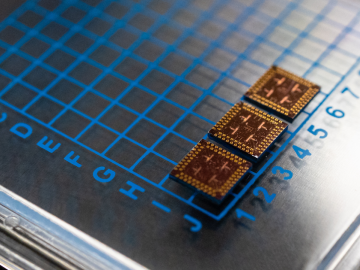
Scientists at have experimentally demonstrated a novel cryogenic, or low temperature, memory cell circuit design based on coupled arrays of Josephson junctions, a technology that may be faster and more energy efficient than existing memory devices.

Using additive manufacturing, scientists experimenting with tungsten at Oak Ridge National Laboratory hope to unlock new potential of the high-performance heat-transferring material used to protect components from the plasma inside a fusion reactor. Fusion requires hydrogen isotopes to reach millions of degrees.
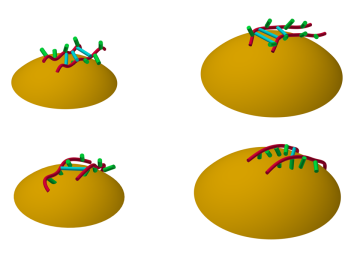
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.
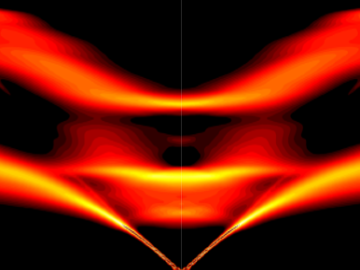
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials
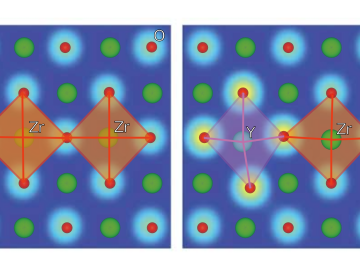
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.

Oak Ridge National Laboratory scientists analyzed more than 50 years of data showing puzzlingly inconsistent trends about corrosion of structural alloys in molten salts and found one factor mattered most—salt purity.
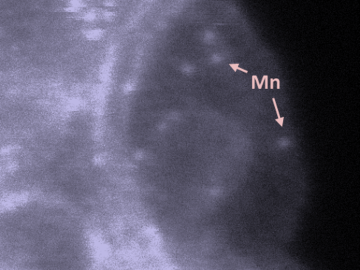
Oak Ridge National Laboratory scientists studying fuel cells as a potential alternative to internal combustion engines used sophisticated electron microscopy to investigate the benefits of replacing high-cost platinum with a lower cost, carbon-nitrogen-manganese-based catalyst.


