Filter News
Area of Research
- (-) Materials (226)
- Advanced Manufacturing (14)
- Biological Systems (15)
- Biology and Environment (42)
- Biology and Soft Matter (1)
- Building Technologies (3)
- Chemistry and Physics at Interfaces (4)
- Clean Energy (187)
- Climate and Environmental Systems (2)
- Computational Biology (4)
- Computational Engineering (2)
- Computer Science (6)
- Electricity and Smart Grid (1)
- Energy Frontier Research Centers (7)
- Energy Sciences (3)
- Fossil Energy (2)
- Fuel Cycle Science and Technology (1)
- Functional Materials for Energy (8)
- Fusion and Fission (19)
- Fusion Energy (2)
- Geographic Information Science and Technology (2)
- Isotope Development and Production (1)
- Isotopes (10)
- Materials Characterization (2)
- Materials for Computing (13)
- Materials Synthesis from Atoms to Systems (5)
- Materials Under Extremes (5)
- National Security (25)
- Neutron Science (80)
- Nuclear Science and Technology (27)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Nuclear Systems Technology (1)
- Quantum Condensed Matter (1)
- Quantum information Science (2)
- Reactor Technology (1)
- Sensors and Controls (1)
- Supercomputing (115)
- Transportation Systems (5)
News Type
News Topics
- 3-D Printing/Advanced Manufacturing (13)
- Advanced Reactors (2)
- Artificial Intelligence (5)
- Bioenergy (8)
- Biology (4)
- Biomedical (3)
- Buildings (3)
- Chemical Sciences (21)
- Climate Change (5)
- Composites (3)
- Computer Science (8)
- Coronavirus (2)
- Critical Materials (8)
- Cybersecurity (3)
- Decarbonization (5)
- Energy Storage (20)
- Environment (8)
- Exascale Computing (1)
- Frontier (3)
- Fusion (2)
- Grid (3)
- High-Performance Computing (3)
- Irradiation (1)
- Isotopes (5)
- ITER (1)
- Machine Learning (3)
- Materials (43)
- Materials Science (37)
- Microscopy (14)
- Molten Salt (2)
- Nanotechnology (21)
- National Security (3)
- Net Zero (1)
- Neutron Science (19)
- Nuclear Energy (3)
- Partnerships (9)
- Physics (14)
- Polymers (6)
- Quantum Computing (1)
- Quantum Science (10)
- Renewable Energy (1)
- Security (1)
- Simulation (1)
- Summit (1)
- Sustainable Energy (8)
- Transformational Challenge Reactor (1)
- Transportation (5)
Media Contacts
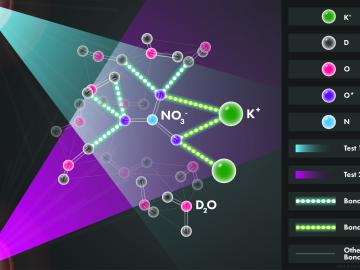
Scientists at the Department of Energy’s Oak Ridge National Laboratory used neutrons, isotopes and simulations to “see” the atomic structure of a saturated solution and found evidence supporting one of two competing hypotheses about how ions come

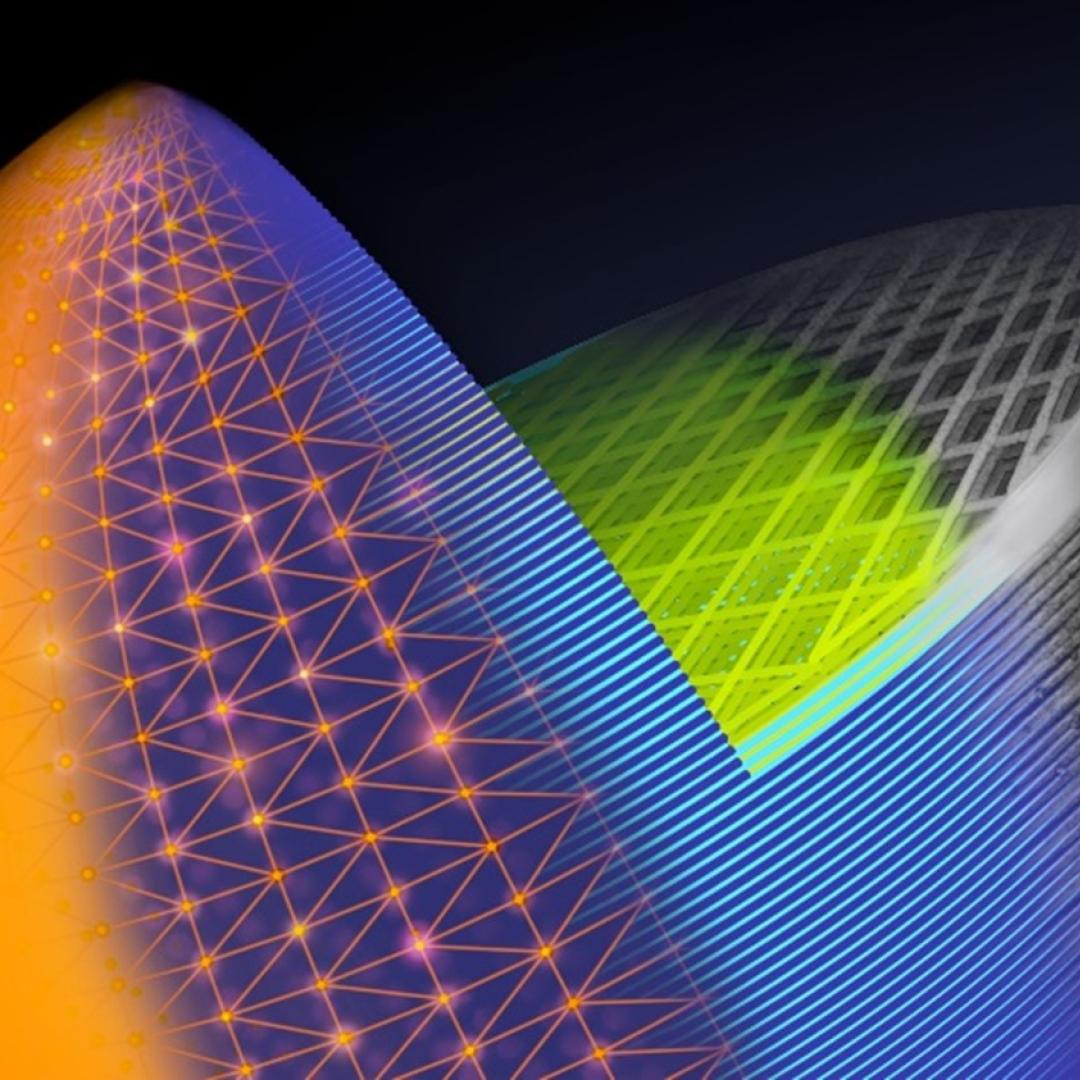
A unique combination of imaging tools and atomic-level simulations has allowed a team led by the Department of Energy’s Oak Ridge National Laboratory to solve a longstanding debate about the properties of a promising material that can harvest energy from light. Th...

Chemists at the Department of Energy’s Oak Ridge National Laboratory have demonstrated a practical, energy-efficient method of capturing carbon dioxide (CO2) directly from air. They report their findings in Nature Energy. If deployed at large scale and coupled to geo...
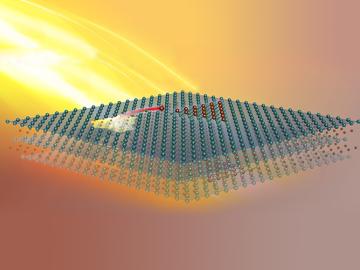
Scientists at the Department of Energy’s Oak Ridge National Laboratory induced a two-dimensional material to cannibalize itself for atomic “building blocks” from which stable structures formed. The findings, reported in Nature Communications, provide insights that ...

Orlando Rios, a researcher at the Department of Energy’s Oak Ridge National Laboratory, has been named a winner of a HENAAC Award, given by Great Minds in STEM, a nonprofit organization that focuses on STEM education awareness programs

Kimberly Jeskie and Michelle Kidder of the Department of Energy’s Oak Ridge National Laboratory have been named 2018 American Chemical Society (ACS) fellows. ACS is a professional organization focused on the advancement of chemistry enterprise and the improvement of...

StealthCo, Inc., an Oak Ridge, Tenn.-based firm doing business as Stealth Mark, has exclusively licensed an invisible micro-taggant from the Department of Energy’s Oak Ridge National Laboratory. The anticounterfeiting technology features a novel materials coding system that uses an infrared marker for identification.



