Filter News
Area of Research
- (-) Materials (86)
- Advanced Manufacturing (10)
- Biology and Environment (33)
- Building Technologies (2)
- Clean Energy (82)
- Computational Biology (1)
- Computational Engineering (3)
- Computer Science (10)
- Electricity and Smart Grid (1)
- Energy Frontier Research Centers (1)
- Energy Sciences (1)
- Functional Materials for Energy (1)
- Fusion and Fission (6)
- Fusion Energy (3)
- Isotope Development and Production (1)
- Isotopes (11)
- Materials Characterization (1)
- Materials for Computing (14)
- Materials Under Extremes (1)
- Mathematics (1)
- National Security (19)
- Neutron Science (28)
- Nuclear Science and Technology (6)
- Quantum information Science (2)
- Supercomputing (41)
- Transportation Systems (1)
News Type
News Topics
- (-) Artificial Intelligence (5)
- (-) Biomedical (5)
- (-) Clean Water (1)
- (-) Cybersecurity (3)
- (-) Isotopes (7)
- (-) Materials Science (56)
- (-) Nanotechnology (29)
- (-) Sustainable Energy (11)
- 3-D Printing/Advanced Manufacturing (19)
- Advanced Reactors (3)
- Bioenergy (9)
- Biology (4)
- Buildings (4)
- Chemical Sciences (25)
- Climate Change (5)
- Composites (7)
- Computer Science (9)
- Coronavirus (3)
- Critical Materials (13)
- Decarbonization (6)
- Energy Storage (27)
- Environment (9)
- Exascale Computing (1)
- Frontier (3)
- Fusion (4)
- Grid (3)
- High-Performance Computing (3)
- Irradiation (1)
- ITER (1)
- Machine Learning (3)
- Materials (55)
- Microscopy (20)
- Molten Salt (3)
- National Security (3)
- Net Zero (1)
- Neutron Science (23)
- Nuclear Energy (6)
- Partnerships (9)
- Physics (16)
- Polymers (12)
- Quantum Computing (2)
- Quantum Science (11)
- Renewable Energy (1)
- Security (1)
- Simulation (1)
- Space Exploration (1)
- Summit (1)
- Transformational Challenge Reactor (1)
- Transportation (11)
Media Contacts
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and

Scientists have tested a novel heat-shielding graphite foam, originally created at Oak Ridge National Laboratory, at Germany’s Wendelstein 7-X stellarator with promising results for use in plasma-facing components of fusion reactors.

OAK RIDGE, Tenn., Feb. 8, 2019—The Department of Energy’s Oak Ridge National Laboratory has named Sean Hearne director of the Center for Nanophase Materials Sciences. The center is a DOE Office of Science User Facility that brings world-leading resources and capabilities to the nanoscience resear...

OAK RIDGE, Tenn., Jan. 31, 2019—A new electron microscopy technique that detects the subtle changes in the weight of proteins at the nanoscale—while keeping the sample intact—could open a new pathway for deeper, more comprehensive studies of the basic building blocks of life.
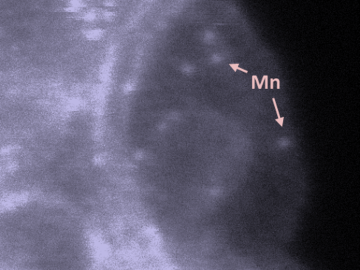
Oak Ridge National Laboratory scientists studying fuel cells as a potential alternative to internal combustion engines used sophisticated electron microscopy to investigate the benefits of replacing high-cost platinum with a lower cost, carbon-nitrogen-manganese-based catalyst.
Physicists turned to the “doubly magic” tin isotope Sn-132, colliding it with a target at Oak Ridge National Laboratory to assess its properties as it lost a neutron to become Sn-131.
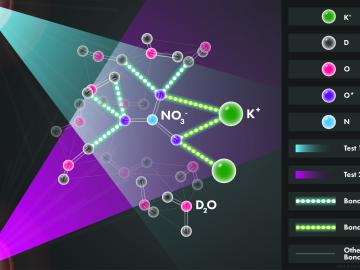
Scientists at the Department of Energy’s Oak Ridge National Laboratory used neutrons, isotopes and simulations to “see” the atomic structure of a saturated solution and found evidence supporting one of two competing hypotheses about how ions come

An Oak Ridge National Laboratory-led team used a scanning transmission electron microscope to selectively position single atoms below a crystal’s surface for the first time.
Scientists at the Department of Energy’s Oak Ridge National Laboratory induced a two-dimensional material to cannibalize itself for atomic “building blocks” from which stable structures formed. The findings, reported in Nature Communications, provide insights that ...
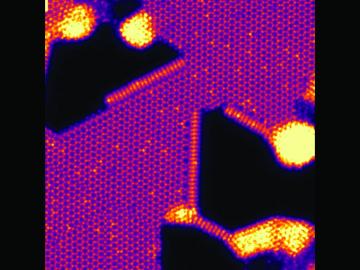
An Oak Ridge National Laboratory–led team has learned how to engineer tiny pores embellished with distinct edge structures inside atomically-thin two-dimensional, or 2D, crystals. The 2D crystals are envisioned as stackable building blocks for ultrathin electronics and other advance...