Filter News
Area of Research
News Topics
- (-) Artificial Intelligence (4)
- (-) Biomedical (7)
- 3-D Printing/Advanced Manufacturing (15)
- Advanced Reactors (1)
- Big Data (1)
- Bioenergy (9)
- Biology (8)
- Biotechnology (1)
- Buildings (2)
- Chemical Sciences (20)
- Climate Change (5)
- Composites (3)
- Computer Science (11)
- Coronavirus (7)
- Critical Materials (8)
- Cybersecurity (4)
- Decarbonization (5)
- Energy Storage (20)
- Environment (10)
- Exascale Computing (1)
- Frontier (3)
- Fusion (3)
- Grid (2)
- High-Performance Computing (3)
- Isotopes (5)
- ITER (1)
- Machine Learning (2)
- Materials (40)
- Materials Science (39)
- Microscopy (12)
- Molten Salt (2)
- Nanotechnology (22)
- National Security (3)
- Net Zero (1)
- Neutron Science (42)
- Nuclear Energy (3)
- Partnerships (8)
- Physics (16)
- Polymers (6)
- Quantum Computing (1)
- Quantum Science (11)
- Renewable Energy (1)
- Security (2)
- Space Exploration (1)
- Summit (4)
- Sustainable Energy (8)
- Transformational Challenge Reactor (1)
- Transportation (6)
Media Contacts

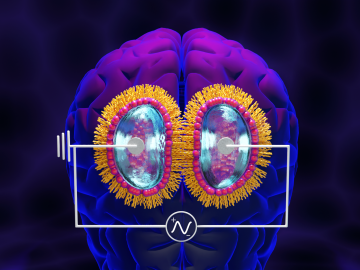
Scientist-inventors from ORNL will present seven new technologies during the Technology Innovation Showcase on Friday, July 14, from 8 a.m.–4 p.m. at the Joint Institute for Computational Sciences on ORNL’s campus.
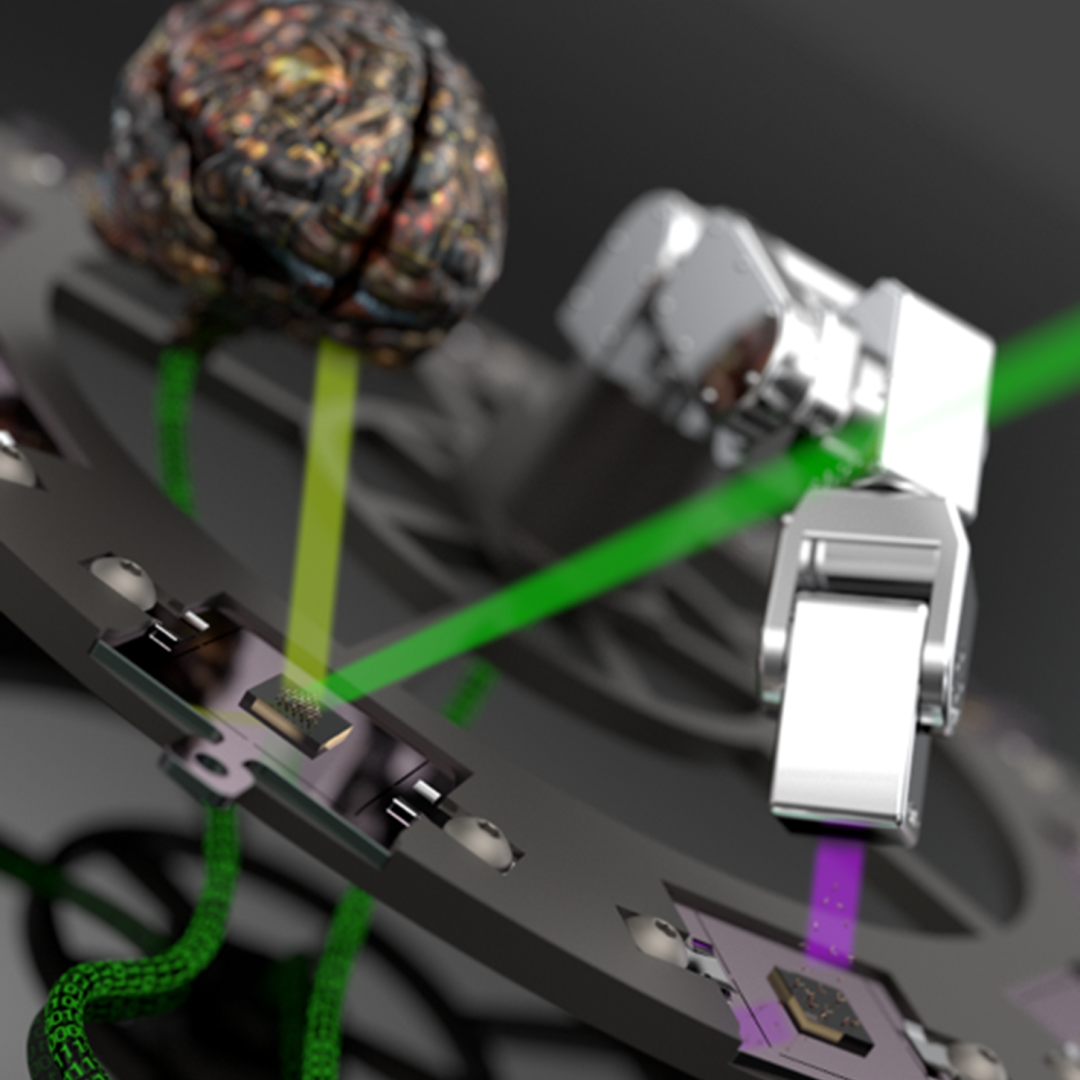
While studying how bio-inspired materials might inform the design of next-generation computers, scientists at ORNL achieved a first-of-its-kind result that could have big implications for both edge computing and human health.

ORNL scientists will present new technologies available for licensing during the annual Technology Innovation Showcase. The event is 9 a.m. to 3 p.m. Thursday, June 16, at the Manufacturing Demonstration Facility at ORNL’s Hardin Valley campus.
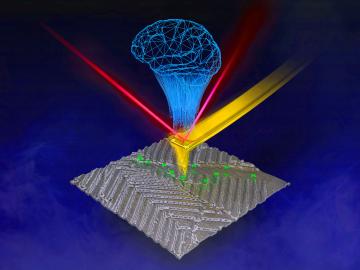
Researchers at ORNL are teaching microscopes to drive discoveries with an intuitive algorithm, developed at the lab’s Center for Nanophase Materials Sciences, that could guide breakthroughs in new materials for energy technologies, sensing and computing.

ORNL, TVA and TNECD were recognized by the Federal Laboratory Consortium for their impactful partnership that resulted in a record $2.3 billion investment by Ultium Cells, a General Motors and LG Energy Solution joint venture, to build a battery cell manufacturing plant in Spring Hill, Tennessee.
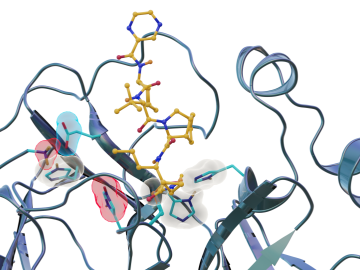
Scientists have found new, unexpected behaviors when SARS-CoV-2 – the virus that causes COVID-19 – encounters drugs known as inhibitors, which bind to certain components of the virus and block its ability to reproduce.

Six scientists at the Department of Energy’s Oak Ridge National Laboratory were named Battelle Distinguished Inventors, in recognition of obtaining 14 or more patents during their careers at the lab.
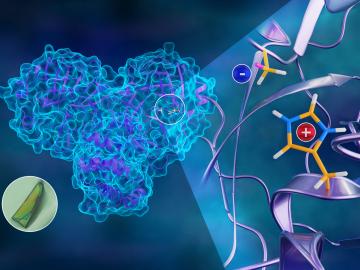
To better understand how the novel coronavirus behaves and how it can be stopped, scientists have completed a three-dimensional map that reveals the location of every atom in an enzyme molecule critical to SARS-CoV-2 reproduction.
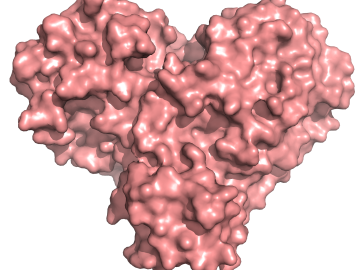
A team of researchers has performed the first room-temperature X-ray measurements on the SARS-CoV-2 main protease — the enzyme that enables the virus to reproduce.
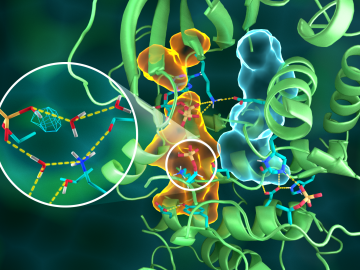
OAK RIDGE, Tenn., March 20, 2019—Direct observations of the structure and catalytic mechanism of a prototypical kinase enzyme—protein kinase A or PKA—will provide researchers and drug developers with significantly enhanced abilities to understand and treat fatal diseases and neurological disorders such as cancer, diabetes, and cystic fibrosis.