
Filter News
Area of Research
- (-) Materials (66)
- Advanced Manufacturing (2)
- Biology and Environment (17)
- Clean Energy (80)
- Computational Engineering (2)
- Computer Science (6)
- Electricity and Smart Grid (1)
- Energy Frontier Research Centers (1)
- Energy Sciences (1)
- Fusion and Fission (12)
- Fusion Energy (8)
- Isotopes (1)
- Materials for Computing (10)
- Mathematics (1)
- National Security (9)
- Neutron Science (12)
- Nuclear Science and Technology (5)
- Quantum information Science (1)
- Sensors and Controls (1)
- Supercomputing (20)
News Type
News Topics
- (-) Clean Water (1)
- (-) Energy Storage (26)
- (-) Fusion (4)
- (-) Grid (2)
- (-) Machine Learning (2)
- (-) Molten Salt (3)
- (-) Nanotechnology (29)
- (-) Polymers (12)
- 3-D Printing/Advanced Manufacturing (19)
- Advanced Reactors (2)
- Artificial Intelligence (4)
- Bioenergy (9)
- Biology (4)
- Biomedical (5)
- Buildings (3)
- Chemical Sciences (24)
- Climate Change (5)
- Composites (7)
- Computer Science (9)
- Coronavirus (3)
- Critical Materials (13)
- Cybersecurity (3)
- Decarbonization (5)
- Environment (8)
- Exascale Computing (1)
- Frontier (2)
- High-Performance Computing (2)
- Isotopes (7)
- ITER (1)
- Materials (50)
- Materials Science (54)
- Microscopy (18)
- National Security (3)
- Net Zero (1)
- Neutron Science (22)
- Nuclear Energy (5)
- Partnerships (8)
- Physics (16)
- Quantum Computing (2)
- Quantum Science (11)
- Renewable Energy (1)
- Security (1)
- Space Exploration (1)
- Summit (1)
- Sustainable Energy (10)
- Transformational Challenge Reactor (1)
- Transportation (10)
Media Contacts
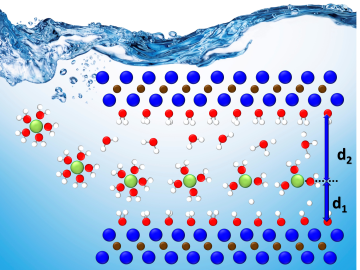
A team led by Oak Ridge National Laboratory developed a novel, integrated approach to track energy-transporting ions within an ultra-thin material, which could unlock its energy storage potential leading toward faster charging, longer-lasting devices.
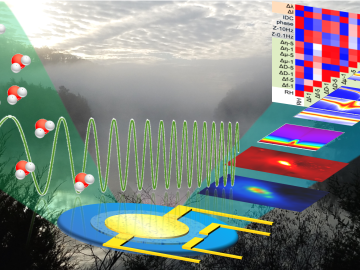
An all-in-one experimental platform developed at Oak Ridge National Laboratory’s Center for Nanophase Materials Sciences accelerates research on promising materials for future technologies.
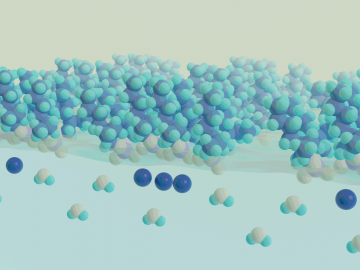
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.

Five researchers at the Department of Energy’s Oak Ridge National Laboratory have been named ORNL Corporate Fellows in recognition of significant career accomplishments and continued leadership in their scientific fields.
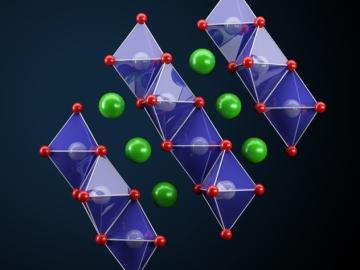
Oak Ridge National Laboratory scientists seeking the source of charge loss in lithium-ion batteries demonstrated that coupling a thin-film cathode with a solid electrolyte is a rapid way to determine the root cause.
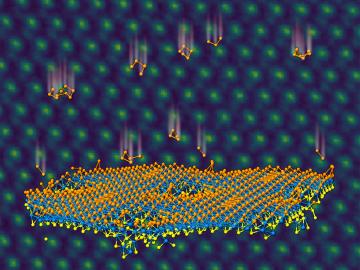
An ORNL team used a simple process to implant atoms precisely into the top layers of ultra-thin crystals, yielding two-sided structures with different chemical compositions.
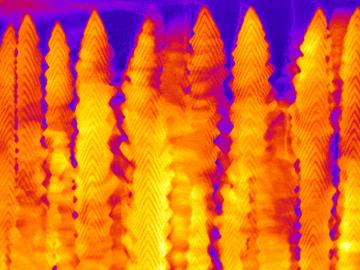
A team led by the Department of Energy’s Oak Ridge National Laboratory synthesized a tiny structure with high surface area and discovered how its unique architecture drives ions across interfaces to transport energy or information.
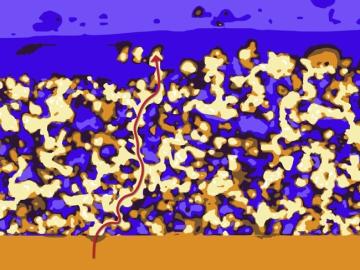
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
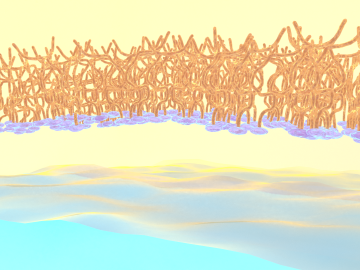
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.

Energy storage startup SPARKZ Inc. has exclusively licensed five battery technologies from the Department of Energy’s Oak Ridge National Laboratory designed to eliminate cobalt metal in lithium-ion batteries. The advancement is aimed at accelerating the production of electric vehicles and energy storage solutions for the power grid.


