
Filter News
Area of Research
- (-) Materials (61)
- Advanced Manufacturing (3)
- Biology and Environment (12)
- Clean Energy (65)
- Computational Engineering (1)
- Computer Science (10)
- Energy Sciences (1)
- Fuel Cycle Science and Technology (1)
- Fusion and Fission (13)
- Fusion Energy (6)
- Isotope Development and Production (1)
- Isotopes (3)
- Materials for Computing (8)
- National Security (14)
- Neutron Science (69)
- Nuclear Science and Technology (18)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Quantum information Science (4)
- Sensors and Controls (1)
- Supercomputing (40)
News Type
News Topics
- (-) Artificial Intelligence (4)
- (-) Energy Storage (26)
- (-) Machine Learning (2)
- (-) Neutron Science (22)
- (-) Nuclear Energy (5)
- (-) Quantum Science (11)
- (-) Security (1)
- (-) Transformational Challenge Reactor (1)
- 3-D Printing/Advanced Manufacturing (19)
- Advanced Reactors (2)
- Bioenergy (9)
- Biology (4)
- Biomedical (5)
- Buildings (3)
- Chemical Sciences (24)
- Clean Water (1)
- Climate Change (5)
- Composites (7)
- Computer Science (9)
- Coronavirus (3)
- Critical Materials (13)
- Cybersecurity (3)
- Decarbonization (5)
- Environment (8)
- Exascale Computing (1)
- Frontier (2)
- Fusion (4)
- Grid (2)
- High-Performance Computing (2)
- Isotopes (7)
- ITER (1)
- Materials (50)
- Materials Science (54)
- Microscopy (18)
- Molten Salt (3)
- Nanotechnology (29)
- National Security (3)
- Net Zero (1)
- Partnerships (8)
- Physics (16)
- Polymers (12)
- Quantum Computing (2)
- Renewable Energy (1)
- Space Exploration (1)
- Summit (1)
- Sustainable Energy (10)
- Transportation (10)
Media Contacts
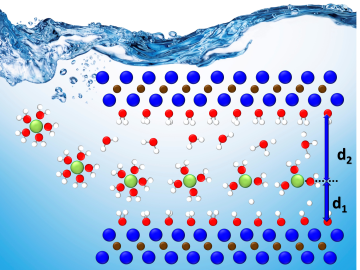
A team led by Oak Ridge National Laboratory developed a novel, integrated approach to track energy-transporting ions within an ultra-thin material, which could unlock its energy storage potential leading toward faster charging, longer-lasting devices.

Five researchers at the Department of Energy’s Oak Ridge National Laboratory have been named ORNL Corporate Fellows in recognition of significant career accomplishments and continued leadership in their scientific fields.
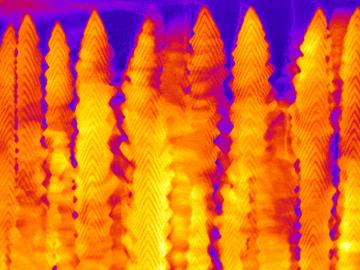
Oak Ridge National Laboratory scientists seeking the source of charge loss in lithium-ion batteries demonstrated that coupling a thin-film cathode with a solid electrolyte is a rapid way to determine the root cause.
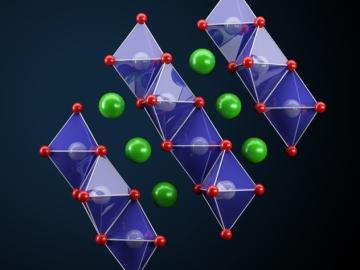
A team led by the Department of Energy’s Oak Ridge National Laboratory synthesized a tiny structure with high surface area and discovered how its unique architecture drives ions across interfaces to transport energy or information.
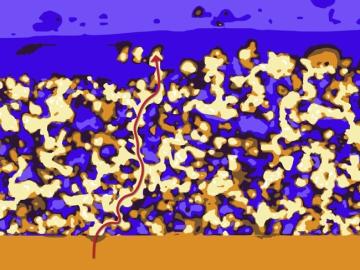
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.

Energy storage startup SPARKZ Inc. has exclusively licensed five battery technologies from the Department of Energy’s Oak Ridge National Laboratory designed to eliminate cobalt metal in lithium-ion batteries. The advancement is aimed at accelerating the production of electric vehicles and energy storage solutions for the power grid.

Using additive manufacturing, scientists experimenting with tungsten at Oak Ridge National Laboratory hope to unlock new potential of the high-performance heat-transferring material used to protect components from the plasma inside a fusion reactor. Fusion requires hydrogen isotopes to reach millions of degrees.
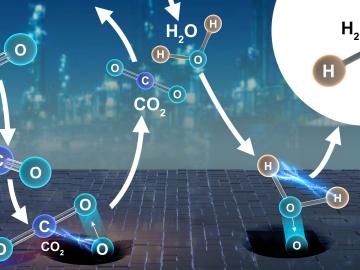
Collaborators at the Department of Energy’s Oak Ridge National Laboratory and U.S. universities used neutron scattering and other advanced characterization techniques to study how a prominent catalyst enables the “water-gas shift” reaction to purify and generate hydrogen at industrial scale.
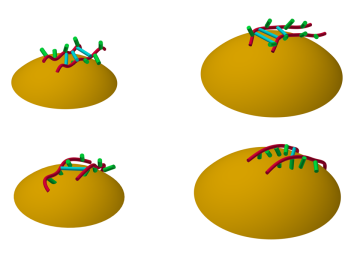
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Researchers have pioneered a new technique using pressure to manipulate magnetism in thin film materials used to enhance performance in electronic devices.


