
Filter News
Area of Research
- (-) Materials (43)
- Biology and Environment (34)
- Clean Energy (90)
- Climate and Environmental Systems (2)
- Computational Biology (1)
- Computational Engineering (2)
- Computer Science (2)
- Energy Sciences (1)
- Fusion and Fission (3)
- Isotopes (10)
- Materials for Computing (8)
- Mathematics (1)
- National Security (6)
- Neutron Science (15)
- Nuclear Science and Technology (6)
- Supercomputing (25)
- Transportation Systems (2)
News Type
News Topics
- (-) Biomedical (5)
- (-) Climate Change (5)
- (-) Coronavirus (3)
- (-) Energy Storage (26)
- (-) Isotopes (7)
- (-) Molten Salt (3)
- (-) Transportation (10)
- 3-D Printing/Advanced Manufacturing (19)
- Advanced Reactors (2)
- Artificial Intelligence (4)
- Bioenergy (9)
- Biology (4)
- Buildings (3)
- Chemical Sciences (24)
- Clean Water (1)
- Composites (7)
- Computer Science (9)
- Critical Materials (13)
- Cybersecurity (3)
- Decarbonization (5)
- Environment (8)
- Exascale Computing (1)
- Frontier (2)
- Fusion (4)
- Grid (2)
- High-Performance Computing (2)
- ITER (1)
- Machine Learning (2)
- Materials (50)
- Materials Science (54)
- Microscopy (18)
- Nanotechnology (29)
- National Security (3)
- Net Zero (1)
- Neutron Science (22)
- Nuclear Energy (5)
- Partnerships (8)
- Physics (16)
- Polymers (12)
- Quantum Computing (2)
- Quantum Science (11)
- Renewable Energy (1)
- Security (1)
- Space Exploration (1)
- Summit (1)
- Sustainable Energy (10)
- Transformational Challenge Reactor (1)
Media Contacts
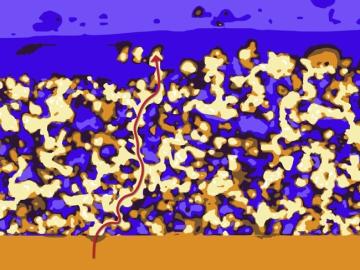
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.

Energy storage startup SPARKZ Inc. has exclusively licensed five battery technologies from the Department of Energy’s Oak Ridge National Laboratory designed to eliminate cobalt metal in lithium-ion batteries. The advancement is aimed at accelerating the production of electric vehicles and energy storage solutions for the power grid.

ORNL and The University of Toledo have entered into a memorandum of understanding for collaborative research.
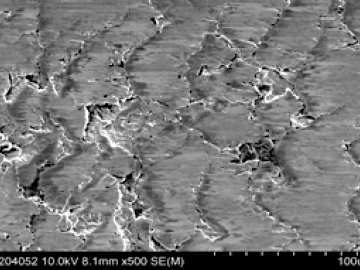
Researchers at Oak Ridge National Laboratory proved that a certain class of ionic liquids, when mixed with commercially available oils, can make gears run more efficiently with less noise and better durability.
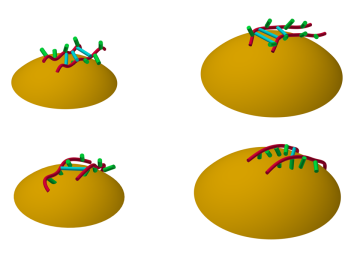
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.
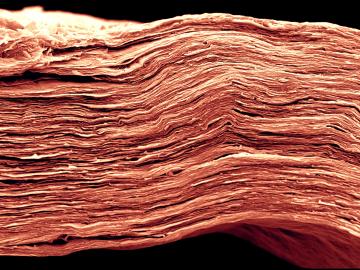

Oak Ridge National Laboratory scientists analyzed more than 50 years of data showing puzzlingly inconsistent trends about corrosion of structural alloys in molten salts and found one factor mattered most—salt purity.

OAK RIDGE, Tenn., Jan. 31, 2019—A new electron microscopy technique that detects the subtle changes in the weight of proteins at the nanoscale—while keeping the sample intact—could open a new pathway for deeper, more comprehensive studies of the basic building blocks of life.
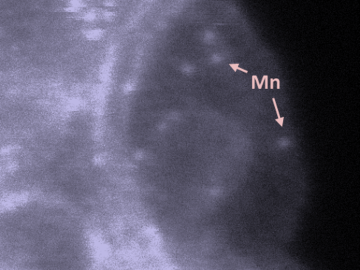
Oak Ridge National Laboratory scientists studying fuel cells as a potential alternative to internal combustion engines used sophisticated electron microscopy to investigate the benefits of replacing high-cost platinum with a lower cost, carbon-nitrogen-manganese-based catalyst.
Physicists turned to the “doubly magic” tin isotope Sn-132, colliding it with a target at Oak Ridge National Laboratory to assess its properties as it lost a neutron to become Sn-131.


