Filter News
Area of Research
- (-) Fusion and Fission (4)
- (-) Materials for Computing (10)
- Advanced Manufacturing (15)
- Biological Systems (2)
- Biology and Environment (32)
- Building Technologies (7)
- Chemical and Engineering Materials (1)
- Clean Energy (152)
- Climate and Environmental Systems (4)
- Computational Biology (1)
- Computational Engineering (2)
- Computer Science (10)
- Electricity and Smart Grid (1)
- Energy Sciences (2)
- Fossil Energy (1)
- Fusion Energy (8)
- Isotope Development and Production (1)
- Isotopes (5)
- Materials (75)
- Mathematics (1)
- National Security (8)
- Neutron Data Analysis and Visualization (2)
- Neutron Science (35)
- Nuclear Science and Technology (18)
- Nuclear Systems Modeling, Simulation and Validation (2)
- Quantum information Science (3)
- Renewable Energy (2)
- Sensors and Controls (2)
- Supercomputing (41)
- Transportation Systems (2)
News Topics
- 3-D Printing/Advanced Manufacturing (2)
- Advanced Reactors (1)
- Biomedical (1)
- Chemical Sciences (2)
- Computer Science (1)
- Coronavirus (2)
- Critical Materials (1)
- Energy Storage (1)
- Fusion (2)
- ITER (2)
- Materials (6)
- Materials Science (6)
- Microscopy (2)
- Nanotechnology (3)
- Neutron Science (1)
- Polymers (1)
- Quantum Science (1)
- Sustainable Energy (2)
- Transportation (1)
Media Contacts
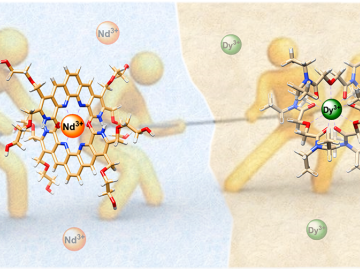
ORNL scientists combined two ligands, or metal-binding molecules, to target light and heavy lanthanides simultaneously for exceptionally efficient separation.
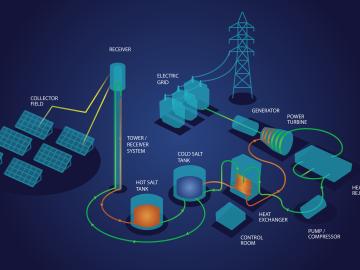
Oak Ridge National Laboratory scientists recently demonstrated a low-temperature, safe route to purifying molten chloride salts that minimizes their ability to corrode metals. This method could make the salts useful for storing energy generated from the sun’s heat.

Oak Ridge National Laboratory researchers collaborated with Iowa State University and RJ Lee Group to demonstrate a safe and effective antiviral coating for N95 masks. The coating destroys the COVID-19-causing coronavirus and could enable reuse of masks made from various fabrics.
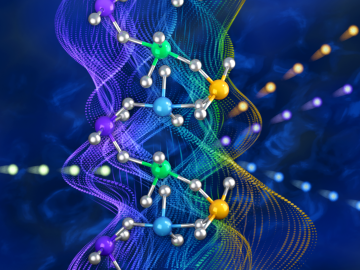
A discovery by Oak Ridge National Laboratory researchers may aid the design of materials that better manage heat.

Staff at Oak Ridge National Laboratory organized transport for a powerful component that is critical to the world’s largest experiment, the international ITER project.

Oak Ridge National Laboratory researchers have developed a new catalyst for converting ethanol into C3+ olefins – the chemical
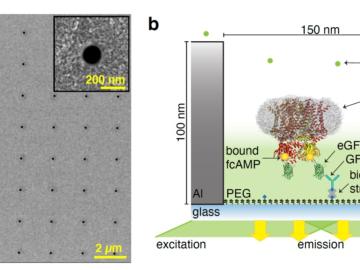
Researchers working with Oak Ridge National Laboratory developed a new method to observe how proteins, at the single-molecule level, bind with other molecules and more accurately pinpoint certain molecular behavior in complex

Equipment and expertise from Oak Ridge National Laboratory will allow scientists studying fusion energy and technologies to acquire crucial data during landmark fusion experiments in Europe.

In a new twist to an existing award-winning ORNL technology, researchers have developed an electrocatalyst that enables water and carbon dioxide to be split and the atoms recombined to form higher weight hydrocarbons for gasoline, diesel and jet fuel.
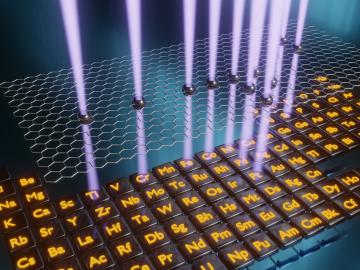
Oak Ridge National Laboratory scientists demonstrated that an electron microscope can be used to selectively remove carbon atoms from graphene’s atomically thin lattice and stitch transition-metal dopant atoms in their place.