Filter News
Area of Research
News Topics
- (-) Critical Materials (12)
- (-) Neutron Science (27)
- (-) Nuclear Energy (18)
- 3-D Printing/Advanced Manufacturing (31)
- Advanced Reactors (13)
- Artificial Intelligence (13)
- Big Data (15)
- Bioenergy (15)
- Biology (17)
- Biomedical (11)
- Biotechnology (3)
- Buildings (18)
- Chemical Sciences (8)
- Clean Water (13)
- Climate Change (21)
- Composites (9)
- Computer Science (39)
- Coronavirus (11)
- Cybersecurity (3)
- Decarbonization (8)
- Energy Storage (31)
- Environment (43)
- Exascale Computing (1)
- Frontier (1)
- Fusion (9)
- Grid (20)
- High-Performance Computing (11)
- Hydropower (6)
- Irradiation (2)
- Isotopes (5)
- ITER (3)
- Machine Learning (10)
- Materials (35)
- Materials Science (33)
- Mathematics (1)
- Mercury (3)
- Microscopy (11)
- Molten Salt (5)
- Nanotechnology (12)
- National Security (3)
- Net Zero (1)
- Partnerships (1)
- Physics (4)
- Polymers (9)
- Quantum Computing (4)
- Quantum Science (10)
- Security (1)
- Simulation (6)
- Space Exploration (10)
- Statistics (1)
- Summit (6)
- Sustainable Energy (44)
- Transportation (35)
Media Contacts
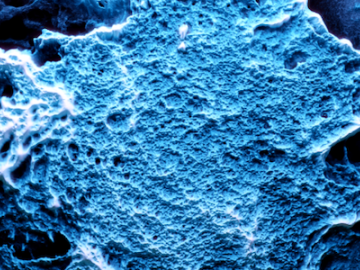
Neutron scattering techniques were used as part of a study of a novel nanoreactor material that grows crystalline hydrogen clathrates, or HCs, capable of storing hydrogen.
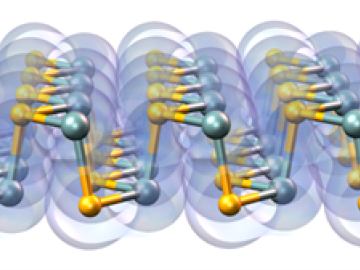
A multi-lab research team led by ORNL's Paul Kent is developing a computer application called QMCPACK to enable precise and reliable predictions of the fundamental properties of materials critical in energy research.

Researchers at Oak Ridge National Laboratory and Momentum Technologies have piloted an industrial-scale process for recycling valuable materials in the millions of tons of e-waste generated annually in the United States.
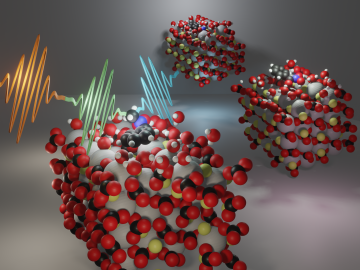
Researchers at Oak Ridge National Laboratory are using state-of-the-art methods to shed light on chemical separations needed to recover rare-earth elements and secure critical materials for clean energy technologies.
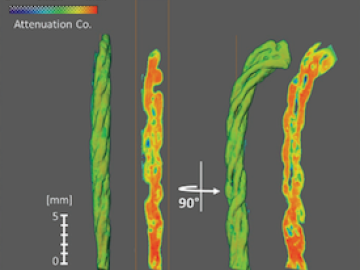
Textile engineering researchers from North Carolina State University used neutrons at Oak Ridge National Laboratory to identify a special wicking mechanism in a type of cotton yarn that allows the fibers to control the flow of liquid across certain strands.
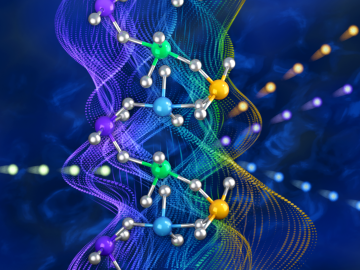
A discovery by Oak Ridge National Laboratory researchers may aid the design of materials that better manage heat.
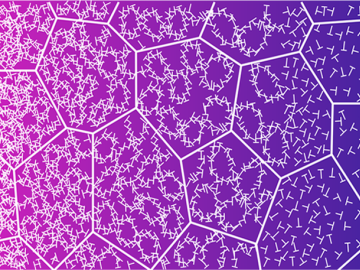

Researchers from NASA’s Jet Propulsion Laboratory and Oak Ridge National Laboratory successfully created amorphous ice, similar to ice in interstellar space and on icy worlds in our solar system. They documented that its disordered atomic behavior is unlike any ice on Earth.
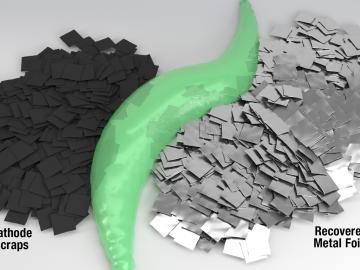
Scientists at Oak Ridge National Laboratory have developed a solvent that results in a more environmentally friendly process to recover valuable materials from used lithium-ion batteries, supports a stable domestic supply chain for new batteries
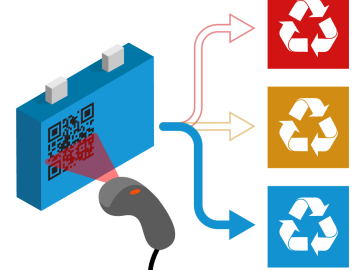
Scientists at Oak Ridge National Laboratory have devised a method to identify the unique chemical makeup of every lithium-ion battery around the world, information that could accelerate recycling, recover critical materials and resolve a growing waste stream.




