Filter News
Area of Research
News Topics
- (-) Chemical Sciences (7)
- (-) Physics (4)
- 3-D Printing/Advanced Manufacturing (30)
- Advanced Reactors (13)
- Artificial Intelligence (13)
- Big Data (15)
- Bioenergy (15)
- Biology (17)
- Biomedical (11)
- Biotechnology (3)
- Buildings (17)
- Clean Water (13)
- Climate Change (20)
- Composites (9)
- Computer Science (39)
- Coronavirus (11)
- Critical Materials (11)
- Cybersecurity (3)
- Decarbonization (7)
- Energy Storage (30)
- Environment (43)
- Exascale Computing (1)
- Frontier (1)
- Fusion (9)
- Grid (20)
- High-Performance Computing (11)
- Hydropower (6)
- Irradiation (2)
- Isotopes (5)
- ITER (3)
- Machine Learning (10)
- Materials (34)
- Materials Science (31)
- Mathematics (1)
- Mercury (3)
- Microscopy (11)
- Molten Salt (5)
- Nanotechnology (12)
- National Security (3)
- Net Zero (1)
- Neutron Science (27)
- Nuclear Energy (18)
- Partnerships (1)
- Polymers (8)
- Quantum Computing (4)
- Quantum Science (10)
- Security (1)
- Simulation (6)
- Space Exploration (10)
- Statistics (1)
- Summit (6)
- Sustainable Energy (42)
- Transportation (35)
Media Contacts
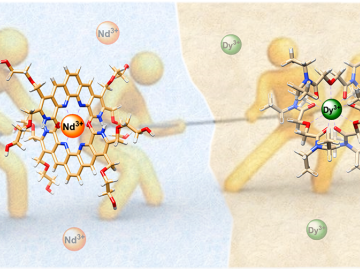
ORNL scientists combined two ligands, or metal-binding molecules, to target light and heavy lanthanides simultaneously for exceptionally efficient separation.
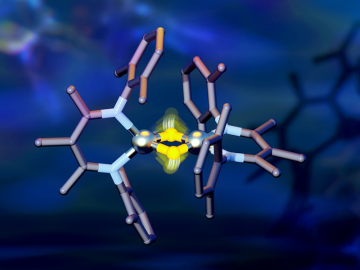
Researchers from Yale University and ORNL collaborated on neutron scattering experiments to study hydrogen atom locations and their effects on iron in a compound similar to those commonly used in industrial catalysts.
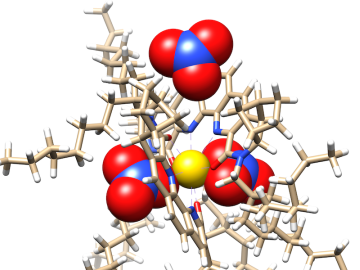
Researchers at ORNL zoomed in on molecules designed to recover critical materials via liquid-liquid extraction — a method used by industry to separate chemically similar elements.
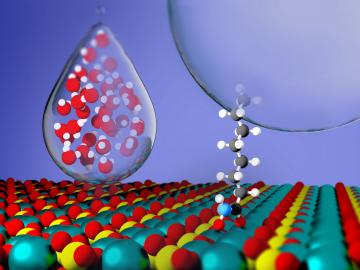
Critical Materials Institute researchers at Oak Ridge National Laboratory and Arizona State University studied the mineral monazite, an important source of rare-earth elements, to enhance methods of recovering critical materials for energy, defense and manufacturing applications.
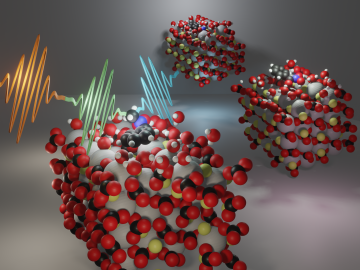
Researchers at Oak Ridge National Laboratory are using state-of-the-art methods to shed light on chemical separations needed to recover rare-earth elements and secure critical materials for clean energy technologies.
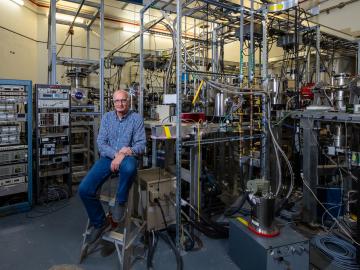
Scientists are using Oak Ridge National Laboratory’s Multicharged Ion Research Facility to simulate the cosmic origin of X-ray emissions resulting when highly charged ions collide with neutral atoms and molecules, such as helium and gaseous hydrogen.

Several electrolyte and thin-film coating technologies, developed at Oak Ridge National Laboratory, have been licensed by BTRY, a battery technology company based in Virginia, to make batteries with increased energy density, at lower cost, and with an improved safety profile in crashes.

Oak Ridge National Laboratory researchers have developed a new catalyst for converting ethanol into C3+ olefins – the chemical

Scientists at Oak Ridge National Laboratory studying quantum communications have discovered a more practical way to share secret messages among three parties, which could ultimately lead to better cybersecurity for the electric grid
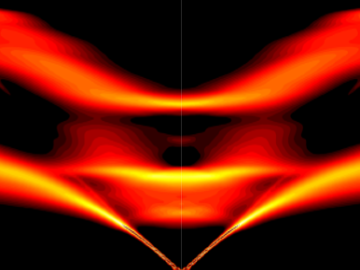
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials