
Filter News
Area of Research
- (-) Clean Energy (186)
- (-) Materials (110)
- (-) National Security (26)
- (-) Neutron Science (35)
- (-) Supercomputing (46)
- Advanced Manufacturing (22)
- Biology and Environment (101)
- Biology and Soft Matter (1)
- Building Technologies (1)
- Climate and Environmental Systems (5)
- Computational Engineering (1)
- Computer Science (4)
- Electricity and Smart Grid (3)
- Functional Materials for Energy (1)
- Fusion and Fission (10)
- Fusion Energy (2)
- Isotope Development and Production (1)
- Isotopes (3)
- Materials Characterization (1)
- Materials for Computing (16)
- Materials Under Extremes (1)
- Mathematics (1)
- Nuclear Science and Technology (7)
- Quantum information Science (1)
- Sensors and Controls (2)
- Transportation Systems (1)
News Topics
- (-) 3-D Printing/Advanced Manufacturing (94)
- (-) Composites (19)
- (-) Environment (85)
- (-) Grid (47)
- (-) Materials Science (102)
- (-) Security (17)
- Advanced Reactors (11)
- Artificial Intelligence (52)
- Big Data (28)
- Bioenergy (38)
- Biology (25)
- Biomedical (33)
- Biotechnology (7)
- Buildings (37)
- Chemical Sciences (34)
- Clean Water (11)
- Climate Change (39)
- Computer Science (119)
- Coronavirus (31)
- Critical Materials (21)
- Cybersecurity (28)
- Decarbonization (38)
- Energy Storage (90)
- Exascale Computing (23)
- Fossil Energy (3)
- Frontier (29)
- Fusion (10)
- High-Performance Computing (44)
- Hydropower (2)
- Irradiation (1)
- Isotopes (13)
- ITER (1)
- Machine Learning (29)
- Materials (106)
- Mathematics (3)
- Mercury (3)
- Microelectronics (1)
- Microscopy (31)
- Molten Salt (3)
- Nanotechnology (47)
- National Security (37)
- Net Zero (4)
- Neutron Science (109)
- Nuclear Energy (30)
- Partnerships (19)
- Physics (36)
- Polymers (23)
- Quantum Computing (20)
- Quantum Science (37)
- Renewable Energy (1)
- Simulation (16)
- Software (1)
- Space Exploration (10)
- Statistics (1)
- Summit (43)
- Sustainable Energy (73)
- Transformational Challenge Reactor (5)
- Transportation (76)
Media Contacts

Kevin Field at the Department of Energy’s Oak Ridge National Laboratory synthesizes and scrutinizes materials for nuclear power systems that must perform safely and efficiently over decades of irradiation.

Alex Roschli is no stranger to finding himself in unique situations. After all, the early career researcher in ORNL’s Manufacturing Systems Research group bears a last name that only 29 other people share in the United States, and he’s certain he’s the only Roschli (a moniker that hails from Switzerland) with the first name Alex.

A residential and commercial tower under development in Brooklyn that is changing the New York City skyline has its roots in research at the Department of Energy’s Oak Ridge National Laboratory.
Higher carbon dioxide levels caused 30 percent more wood growth in young forest stands across the temperate United States over a decade, according to an analysis led by Oak Ridge National Laboratory.
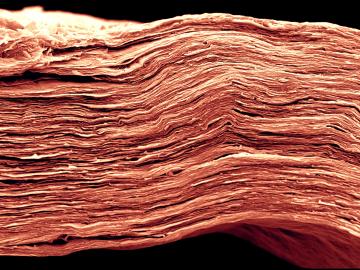
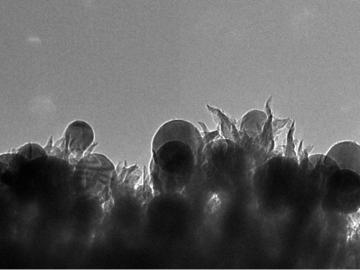
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

The use of lithium-ion batteries has surged in recent years, starting with electronics and expanding into many applications, including the growing electric and hybrid vehicle industry. But the technologies to optimize recycling of these batteries have not kept pace.
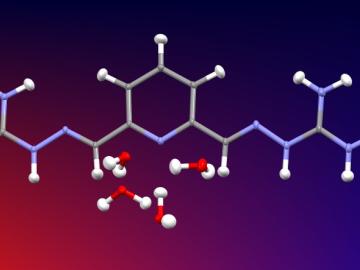
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.



