Filter News
Area of Research
- (-) Materials for Computing (4)
- (-) Neutron Science (6)
- Biology and Environment (7)
- Clean Energy (72)
- Computer Science (2)
- Electricity and Smart Grid (1)
- Energy Sciences (1)
- Functional Materials for Energy (2)
- Fusion and Fission (4)
- Isotopes (1)
- Materials (34)
- National Security (2)
- Supercomputing (8)
News Topics
- (-) Energy Storage (10)
- 3-D Printing/Advanced Manufacturing (10)
- Advanced Reactors (1)
- Artificial Intelligence (6)
- Big Data (2)
- Bioenergy (7)
- Biology (6)
- Biomedical (13)
- Biotechnology (1)
- Chemical Sciences (6)
- Clean Water (2)
- Climate Change (2)
- Composites (2)
- Computer Science (18)
- Coronavirus (11)
- Cybersecurity (1)
- Decarbonization (3)
- Environment (9)
- Fossil Energy (1)
- Frontier (1)
- Fusion (1)
- High-Performance Computing (2)
- Isotopes (1)
- Machine Learning (3)
- Materials (24)
- Materials Science (35)
- Mathematics (1)
- Microscopy (7)
- Nanotechnology (17)
- National Security (3)
- Neutron Science (101)
- Nuclear Energy (3)
- Physics (9)
- Polymers (7)
- Quantum Computing (1)
- Quantum Science (9)
- Security (2)
- Simulation (1)
- Space Exploration (3)
- Summit (6)
- Sustainable Energy (7)
- Transportation (9)
Media Contacts
Currently, the biggest hurdle for electric vehicles, or EVs, is the development of advanced battery technology to extend driving range, safety and reliability.
Researchers at ORNL have developed a new method for producing a key component of lithium-ion batteries. The result is a more affordable battery from a faster, less wasteful process that uses less toxic material.

Researchers at ORNL and the University of Tennessee, Knoxville, discovered a key material needed for fast-charging lithium-ion batteries. The commercially relevant approach opens a potential pathway to improve charging speeds for electric vehicles.

Through a consortium of Department of Energy national laboratories, ORNL scientists are applying their expertise to provide solutions that enable the commercialization of emission-free hydrogen fuel cell technology for heavy-duty
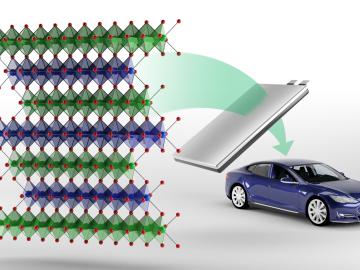
Oak Ridge National Laboratory researchers have developed a new family of cathodes with the potential to replace the costly cobalt-based cathodes typically found in today’s lithium-ion batteries that power electric vehicles and consumer electronics.

Soteria Battery Innovation Group has exclusively licensed and optioned a technology developed by Oak Ridge National Laboratory designed to eliminate thermal runaway in lithium ion batteries due to mechanical damage.

Four research teams from the Department of Energy’s Oak Ridge National Laboratory and their technologies have received 2020 R&D 100 Awards.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.
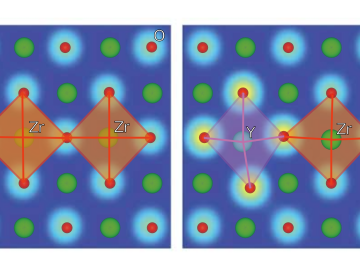
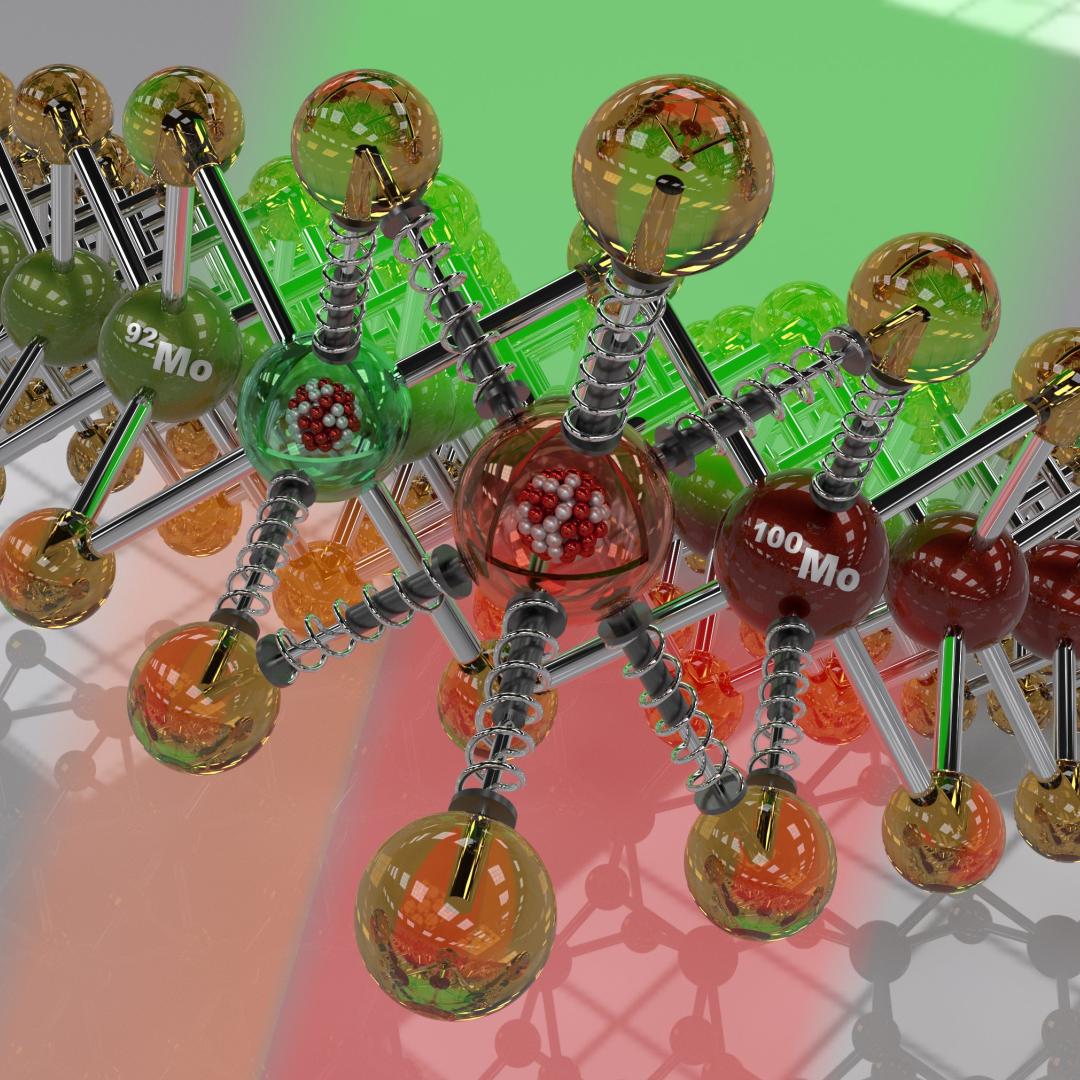
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
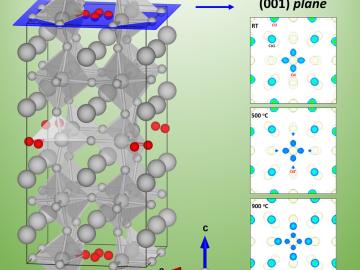
A University of South Carolina research team is investigating the oxygen reduction performance of energy conversion materials called perovskites by using neutron diffraction at Oak Ridge National Laboratory’s Spallation Neutron Source.