Filter News
Area of Research
- (-) Neutron Science (11)
- Advanced Manufacturing (6)
- Biology and Environment (41)
- Building Technologies (2)
- Clean Energy (121)
- Computational Engineering (1)
- Computer Science (7)
- Electricity and Smart Grid (1)
- Energy Sciences (2)
- Functional Materials for Energy (2)
- Fusion and Fission (7)
- Fusion Energy (2)
- Isotopes (1)
- Materials (44)
- Materials for Computing (7)
- National Security (16)
- Nuclear Science and Technology (1)
- Quantum information Science (1)
- Supercomputing (27)
News Type
News Topics
- (-) Energy Storage (6)
- (-) Machine Learning (3)
- (-) Sustainable Energy (2)
- 3-D Printing/Advanced Manufacturing (6)
- Advanced Reactors (1)
- Artificial Intelligence (6)
- Big Data (2)
- Bioenergy (6)
- Biology (5)
- Biomedical (11)
- Biotechnology (1)
- Chemical Sciences (2)
- Clean Water (2)
- Climate Change (1)
- Composites (1)
- Computer Science (13)
- Coronavirus (8)
- Cybersecurity (1)
- Decarbonization (2)
- Environment (8)
- Fossil Energy (1)
- Frontier (1)
- Fusion (1)
- High-Performance Computing (2)
- Materials (14)
- Materials Science (23)
- Mathematics (1)
- Microscopy (3)
- Nanotechnology (10)
- National Security (2)
- Neutron Science (99)
- Nuclear Energy (3)
- Physics (9)
- Polymers (1)
- Quantum Computing (1)
- Quantum Science (7)
- Security (2)
- Space Exploration (3)
- Summit (6)
- Transportation (5)
Media Contacts
Currently, the biggest hurdle for electric vehicles, or EVs, is the development of advanced battery technology to extend driving range, safety and reliability.

Neutron experiments can take days to complete, requiring researchers to work long shifts to monitor progress and make necessary adjustments. But thanks to advances in artificial intelligence and machine learning, experiments can now be done remotely and in half the time.

Like most scientists, Chengping Chai is not content with the surface of things: He wants to probe beyond to learn what’s really going on. But in his case, he is literally building a map of the world beneath, using seismic and acoustic data that reveal when and where the earth moves.
Researchers at ORNL have developed a new method for producing a key component of lithium-ion batteries. The result is a more affordable battery from a faster, less wasteful process that uses less toxic material.

Researchers at ORNL and the University of Tennessee, Knoxville, discovered a key material needed for fast-charging lithium-ion batteries. The commercially relevant approach opens a potential pathway to improve charging speeds for electric vehicles.

Five researchers at the Department of Energy’s Oak Ridge National Laboratory have been named ORNL Corporate Fellows in recognition of significant career accomplishments and continued leadership in their scientific fields.
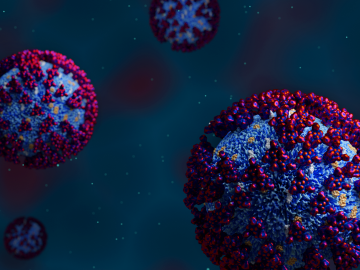
In the race to identify solutions to the COVID-19 pandemic, researchers at the Department of Energy’s Oak Ridge National Laboratory are joining the fight by applying expertise in computational science, advanced manufacturing, data science and neutron science.

ORNL computer scientist Catherine Schuman returned to her alma mater, Harriman High School, to lead Hour of Code activities and talk to students about her job as a researcher.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.
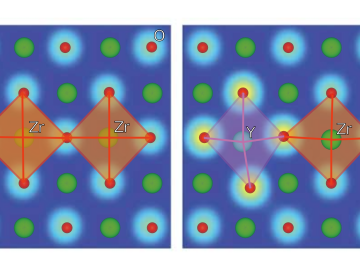
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.