Filter News
Area of Research
- (-) Neutron Science (11)
- Advanced Manufacturing (2)
- Biology and Environment (15)
- Biology and Soft Matter (1)
- Clean Energy (87)
- Computer Science (2)
- Electricity and Smart Grid (1)
- Energy Sciences (1)
- Fuel Cycle Science and Technology (1)
- Functional Materials for Energy (2)
- Fusion and Fission (32)
- Fusion Energy (10)
- Isotope Development and Production (1)
- Isotopes (4)
- Materials (72)
- Materials for Computing (8)
- National Security (8)
- Nuclear Science and Technology (36)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Supercomputing (15)
News Type
News Topics
- (-) Chemical Sciences (2)
- (-) Energy Storage (6)
- (-) Nuclear Energy (3)
- 3-D Printing/Advanced Manufacturing (6)
- Advanced Reactors (1)
- Artificial Intelligence (6)
- Big Data (2)
- Bioenergy (6)
- Biology (5)
- Biomedical (11)
- Biotechnology (1)
- Clean Water (2)
- Climate Change (1)
- Composites (1)
- Computer Science (13)
- Coronavirus (8)
- Cybersecurity (1)
- Decarbonization (2)
- Environment (8)
- Fossil Energy (1)
- Frontier (1)
- Fusion (1)
- High-Performance Computing (2)
- Machine Learning (3)
- Materials (14)
- Materials Science (23)
- Mathematics (1)
- Microscopy (3)
- Nanotechnology (10)
- National Security (2)
- Neutron Science (99)
- Physics (9)
- Polymers (1)
- Quantum Computing (1)
- Quantum Science (7)
- Security (2)
- Space Exploration (3)
- Summit (6)
- Sustainable Energy (2)
- Transportation (5)
Media Contacts
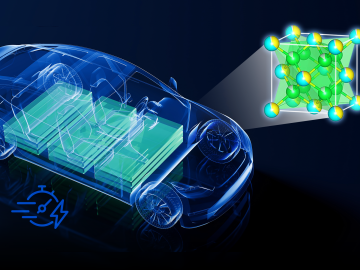
Currently, the biggest hurdle for electric vehicles, or EVs, is the development of advanced battery technology to extend driving range, safety and reliability.

Like most scientists, Chengping Chai is not content with the surface of things: He wants to probe beyond to learn what’s really going on. But in his case, he is literally building a map of the world beneath, using seismic and acoustic data that reveal when and where the earth moves.
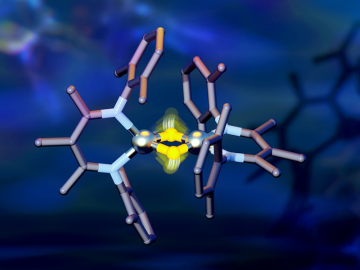
Researchers from Yale University and ORNL collaborated on neutron scattering experiments to study hydrogen atom locations and their effects on iron in a compound similar to those commonly used in industrial catalysts.
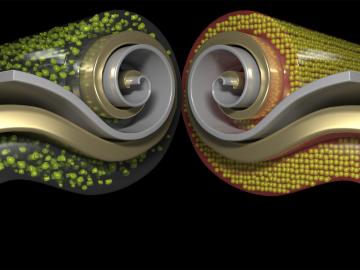
Researchers at ORNL have developed a new method for producing a key component of lithium-ion batteries. The result is a more affordable battery from a faster, less wasteful process that uses less toxic material.

Researchers at ORNL and the University of Tennessee, Knoxville, discovered a key material needed for fast-charging lithium-ion batteries. The commercially relevant approach opens a potential pathway to improve charging speeds for electric vehicles.
At the Department of Energy’s Oak Ridge National Laboratory, scientists use artificial intelligence, or AI, to accelerate the discovery and development of materials for energy and information technologies.
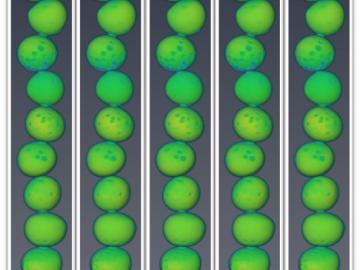
Oak Ridge National Laboratory researchers working on neutron imaging capabilities for nuclear materials have developed a process for seeing the inside of uranium particles – without cutting them open.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
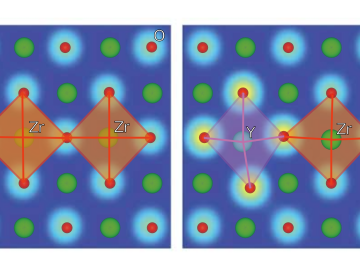
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.




