Filter News
Area of Research
- (-) Neutron Science (8)
- Biology and Environment (15)
- Biology and Soft Matter (1)
- Clean Energy (81)
- Computer Science (2)
- Electricity and Smart Grid (1)
- Energy Sciences (1)
- Functional Materials for Energy (2)
- Fusion and Fission (7)
- Isotopes (1)
- Materials (57)
- Materials for Computing (8)
- National Security (3)
- Supercomputing (11)
News Type
News Topics
- (-) Chemical Sciences (2)
- (-) Energy Storage (6)
- 3-D Printing/Advanced Manufacturing (6)
- Advanced Reactors (1)
- Artificial Intelligence (6)
- Big Data (2)
- Bioenergy (6)
- Biology (5)
- Biomedical (11)
- Biotechnology (1)
- Clean Water (2)
- Climate Change (1)
- Composites (1)
- Computer Science (13)
- Coronavirus (8)
- Cybersecurity (1)
- Decarbonization (2)
- Environment (8)
- Fossil Energy (1)
- Frontier (1)
- Fusion (1)
- High-Performance Computing (2)
- Machine Learning (3)
- Materials (14)
- Materials Science (23)
- Mathematics (1)
- Microscopy (3)
- Nanotechnology (10)
- National Security (2)
- Neutron Science (99)
- Nuclear Energy (3)
- Physics (9)
- Polymers (1)
- Quantum Computing (1)
- Quantum Science (7)
- Security (2)
- Space Exploration (3)
- Summit (6)
- Sustainable Energy (2)
- Transportation (5)
Media Contacts
Currently, the biggest hurdle for electric vehicles, or EVs, is the development of advanced battery technology to extend driving range, safety and reliability.
Researchers from Yale University and ORNL collaborated on neutron scattering experiments to study hydrogen atom locations and their effects on iron in a compound similar to those commonly used in industrial catalysts.
Researchers at ORNL have developed a new method for producing a key component of lithium-ion batteries. The result is a more affordable battery from a faster, less wasteful process that uses less toxic material.

Researchers at ORNL and the University of Tennessee, Knoxville, discovered a key material needed for fast-charging lithium-ion batteries. The commercially relevant approach opens a potential pathway to improve charging speeds for electric vehicles.
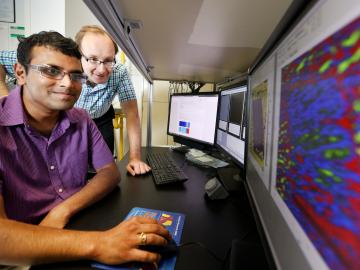
At the Department of Energy’s Oak Ridge National Laboratory, scientists use artificial intelligence, or AI, to accelerate the discovery and development of materials for energy and information technologies.
Two of the researchers who share the Nobel Prize in Chemistry announced Wednesday—John B. Goodenough of the University of Texas at Austin and M. Stanley Whittingham of Binghamton University in New York—have research ties to ORNL.
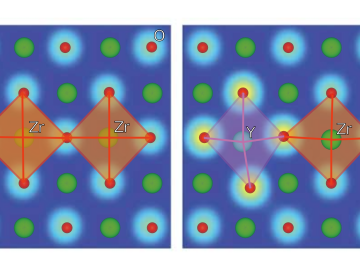
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.
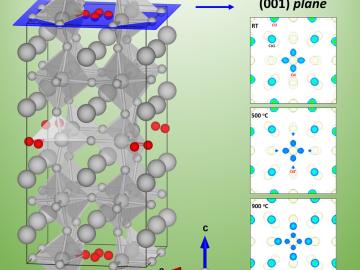
A University of South Carolina research team is investigating the oxygen reduction performance of energy conversion materials called perovskites by using neutron diffraction at Oak Ridge National Laboratory’s Spallation Neutron Source.