
Filter News
Area of Research
- (-) Materials (74)
- (-) National Security (37)
- Advanced Manufacturing (5)
- Biology and Environment (49)
- Building Technologies (1)
- Clean Energy (145)
- Climate and Environmental Systems (1)
- Computational Biology (1)
- Computational Engineering (3)
- Computer Science (16)
- Electricity and Smart Grid (3)
- Functional Materials for Energy (1)
- Fusion and Fission (12)
- Fusion Energy (8)
- Isotopes (1)
- Materials for Computing (16)
- Mathematics (1)
- Neutron Science (110)
- Nuclear Science and Technology (15)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Quantum information Science (6)
- Sensors and Controls (1)
- Supercomputing (122)
- Transportation Systems (2)
News Topics
- (-) Advanced Reactors (5)
- (-) Artificial Intelligence (21)
- (-) Clean Water (3)
- (-) Computer Science (33)
- (-) Coronavirus (6)
- (-) Grid (11)
- (-) Neutron Science (35)
- (-) Transportation (16)
- 3-D Printing/Advanced Manufacturing (25)
- Big Data (7)
- Bioenergy (14)
- Biology (8)
- Biomedical (8)
- Biotechnology (1)
- Buildings (6)
- Chemical Sciences (32)
- Climate Change (9)
- Composites (9)
- Critical Materials (13)
- Cybersecurity (21)
- Decarbonization (9)
- Energy Storage (35)
- Environment (20)
- Exascale Computing (2)
- Frontier (3)
- Fusion (8)
- High-Performance Computing (8)
- Irradiation (1)
- Isotopes (13)
- ITER (1)
- Machine Learning (16)
- Materials (74)
- Materials Science (78)
- Mathematics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (34)
- Net Zero (1)
- Nuclear Energy (21)
- Partnerships (14)
- Physics (29)
- Polymers (17)
- Quantum Computing (3)
- Quantum Science (12)
- Renewable Energy (1)
- Security (11)
- Simulation (2)
- Space Exploration (2)
- Summit (4)
- Sustainable Energy (16)
- Transformational Challenge Reactor (3)
Media Contacts
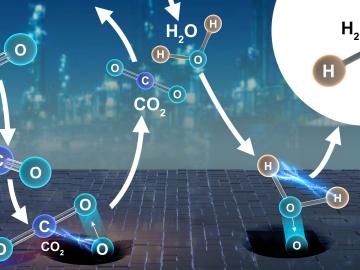
Collaborators at the Department of Energy’s Oak Ridge National Laboratory and U.S. universities used neutron scattering and other advanced characterization techniques to study how a prominent catalyst enables the “water-gas shift” reaction to purify and generate hydrogen at industrial scale.
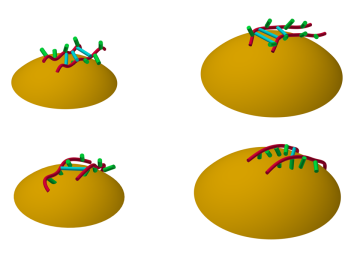
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Researchers have pioneered a new technique using pressure to manipulate magnetism in thin film materials used to enhance performance in electronic devices.
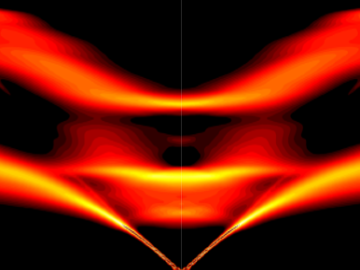
Scientists have discovered a way to alter heat transport in thermoelectric materials, a finding that may ultimately improve energy efficiency as the materials

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
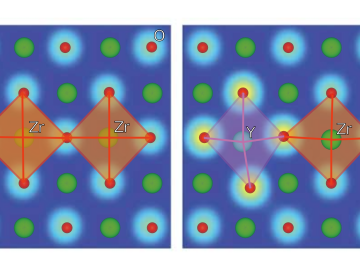
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.

Scientists at the Department of Energy’s Oak Ridge National Laboratory are working to understand both the complex nature of uranium and the various oxide forms it can take during processing steps that might occur throughout the nuclear fuel cycle.
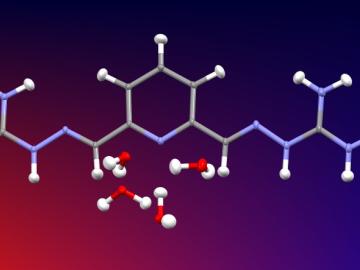
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.
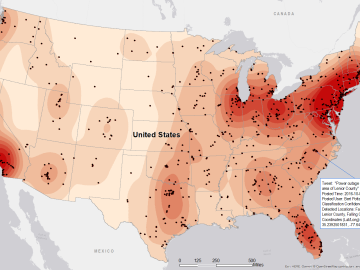
Gleaning valuable data from social platforms such as Twitter—particularly to map out critical location information during emergencies— has become more effective and efficient thanks to Oak Ridge National Laboratory.
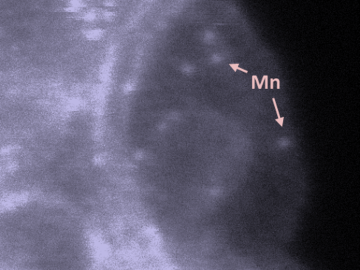
Oak Ridge National Laboratory scientists studying fuel cells as a potential alternative to internal combustion engines used sophisticated electron microscopy to investigate the benefits of replacing high-cost platinum with a lower cost, carbon-nitrogen-manganese-based catalyst.


