Filter News
Area of Research
- (-) Electricity and Smart Grid (1)
- (-) Fusion and Fission (6)
- (-) Materials (75)
- (-) National Security (32)
- Advanced Manufacturing (2)
- Biological Systems (2)
- Biology and Environment (134)
- Biology and Soft Matter (1)
- Building Technologies (1)
- Clean Energy (153)
- Climate and Environmental Systems (5)
- Computational Biology (2)
- Computational Engineering (3)
- Computer Science (16)
- Energy Sciences (1)
- Functional Materials for Energy (2)
- Fusion Energy (2)
- Isotopes (3)
- Materials for Computing (13)
- Mathematics (1)
- Neutron Science (36)
- Nuclear Science and Technology (4)
- Quantum information Science (9)
- Supercomputing (141)
News Topics
- (-) Bioenergy (14)
- (-) Computer Science (35)
- (-) Energy Storage (36)
- (-) Environment (22)
- (-) Machine Learning (16)
- (-) Quantum Science (12)
- (-) Summit (4)
- 3-D Printing/Advanced Manufacturing (28)
- Advanced Reactors (11)
- Artificial Intelligence (22)
- Big Data (7)
- Biology (9)
- Biomedical (9)
- Biotechnology (1)
- Buildings (6)
- Chemical Sciences (34)
- Clean Water (3)
- Climate Change (9)
- Composites (9)
- Coronavirus (6)
- Critical Materials (13)
- Cybersecurity (21)
- Decarbonization (11)
- Exascale Computing (3)
- Fossil Energy (1)
- Frontier (4)
- Fusion (28)
- Grid (14)
- High-Performance Computing (10)
- Irradiation (1)
- Isotopes (14)
- ITER (6)
- Materials (75)
- Materials Science (80)
- Mathematics (1)
- Microelectronics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (34)
- Net Zero (2)
- Neutron Science (36)
- Nuclear Energy (47)
- Partnerships (17)
- Physics (30)
- Polymers (17)
- Quantum Computing (3)
- Renewable Energy (1)
- Security (13)
- Simulation (5)
- Space Exploration (3)
- Sustainable Energy (20)
- Transformational Challenge Reactor (3)
- Transportation (18)
Media Contacts

IDEMIA Identity & Security USA has licensed an advanced optical array developed at Oak Ridge National Laboratory. The portable technology can be used to help identify individuals in challenging outdoor conditions.
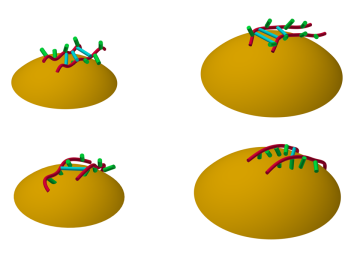
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.
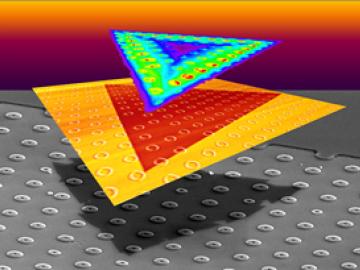
A team led by scientists at the Department of Energy’s Oak Ridge National Laboratory explored how atomically thin two-dimensional (2D) crystals can grow over 3D objects and how the curvature of those objects can stretch and strain the

OAK RIDGE, Tenn., May 7, 2019—Energy Secretary Rick Perry, Congressman Chuck Fleischmann and lab officials today broke ground on a multipurpose research facility that will provide state-of-the-art laboratory space

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
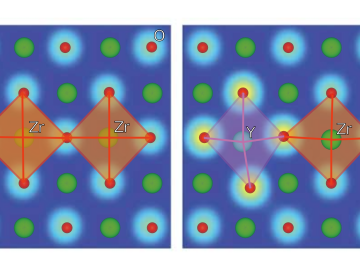
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.

Scientists at the Department of Energy’s Oak Ridge National Laboratory are working to understand both the complex nature of uranium and the various oxide forms it can take during processing steps that might occur throughout the nuclear fuel cycle.
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and
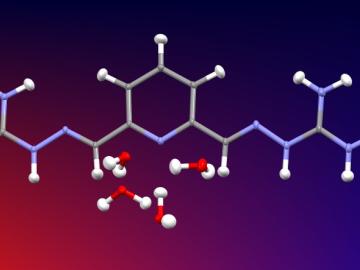
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.


