
Filter News
Area of Research
- (-) Materials (120)
- (-) National Security (31)
- Advanced Manufacturing (10)
- Biological Systems (2)
- Biology and Environment (131)
- Biology and Soft Matter (1)
- Building Technologies (2)
- Clean Energy (160)
- Climate and Environmental Systems (5)
- Computational Biology (1)
- Computational Engineering (3)
- Computer Science (16)
- Electricity and Smart Grid (1)
- Energy Sciences (1)
- Functional Materials for Energy (1)
- Fusion and Fission (27)
- Fusion Energy (13)
- Isotope Development and Production (1)
- Isotopes (3)
- Materials Characterization (1)
- Materials for Computing (21)
- Materials Under Extremes (1)
- Mathematics (1)
- Neutron Science (40)
- Nuclear Science and Technology (15)
- Quantum information Science (7)
- Sensors and Controls (1)
- Supercomputing (114)
- Transportation Systems (1)
News Topics
- (-) Bioenergy (14)
- (-) Clean Water (3)
- (-) Computer Science (33)
- (-) Environment (20)
- (-) Fusion (8)
- (-) Materials Science (78)
- (-) Polymers (17)
- (-) Security (11)
- (-) Sustainable Energy (16)
- 3-D Printing/Advanced Manufacturing (25)
- Advanced Reactors (5)
- Artificial Intelligence (21)
- Big Data (7)
- Biology (8)
- Biomedical (8)
- Biotechnology (1)
- Buildings (6)
- Chemical Sciences (32)
- Climate Change (9)
- Composites (9)
- Coronavirus (6)
- Critical Materials (13)
- Cybersecurity (21)
- Decarbonization (9)
- Energy Storage (35)
- Exascale Computing (2)
- Frontier (3)
- Grid (11)
- High-Performance Computing (8)
- Irradiation (1)
- Isotopes (13)
- ITER (1)
- Machine Learning (16)
- Materials (74)
- Mathematics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (34)
- Net Zero (1)
- Neutron Science (35)
- Nuclear Energy (21)
- Partnerships (14)
- Physics (29)
- Quantum Computing (3)
- Quantum Science (12)
- Renewable Energy (1)
- Simulation (2)
- Space Exploration (2)
- Summit (4)
- Transformational Challenge Reactor (3)
- Transportation (16)
Media Contacts
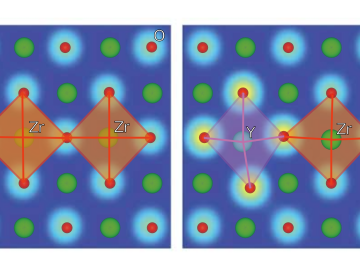
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.

Scientists at the Department of Energy’s Oak Ridge National Laboratory are working to understand both the complex nature of uranium and the various oxide forms it can take during processing steps that might occur throughout the nuclear fuel cycle.

Kevin Field at the Department of Energy’s Oak Ridge National Laboratory synthesizes and scrutinizes materials for nuclear power systems that must perform safely and efficiently over decades of irradiation.
OAK RIDGE, Tenn., March 1, 2019—ReactWell, LLC, has licensed a novel waste-to-fuel technology from the Department of Energy’s Oak Ridge National Laboratory to improve energy conversion methods for cleaner, more efficient oil and gas, chemical and

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.
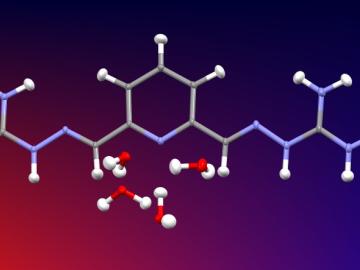
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.

Scientists have tested a novel heat-shielding graphite foam, originally created at Oak Ridge National Laboratory, at Germany’s Wendelstein 7-X stellarator with promising results for use in plasma-facing components of fusion reactors.

OAK RIDGE, Tenn., Feb. 8, 2019—The Department of Energy’s Oak Ridge National Laboratory has named Sean Hearne director of the Center for Nanophase Materials Sciences. The center is a DOE Office of Science User Facility that brings world-leading resources and capabilities to the nanoscience resear...
Scientists at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have developed a process that could remove CO2 from coal-burning power plant emissions in a way that is similar to how soda lime works in scuba diving rebreathers. Their research, published January 31 in...


