Filter News
Area of Research
- (-) Materials (60)
- Biological Systems (1)
- Biology and Environment (111)
- Biology and Soft Matter (1)
- Clean Energy (134)
- Climate and Environmental Systems (5)
- Computational Biology (2)
- Computational Engineering (2)
- Computer Science (4)
- Electricity and Smart Grid (1)
- Energy Sciences (1)
- Functional Materials for Energy (2)
- Fusion and Fission (8)
- Fusion Energy (1)
- Isotopes (7)
- Materials for Computing (6)
- Mathematics (1)
- National Security (13)
- Neutron Science (28)
- Nuclear Science and Technology (3)
- Supercomputing (75)
News Topics
- (-) Biomedical (7)
- (-) Clean Water (3)
- (-) Energy Storage (34)
- (-) Environment (15)
- (-) Partnerships (11)
- (-) Summit (2)
- 3-D Printing/Advanced Manufacturing (23)
- Advanced Reactors (4)
- Artificial Intelligence (9)
- Big Data (2)
- Bioenergy (11)
- Biology (4)
- Buildings (5)
- Chemical Sciences (32)
- Climate Change (5)
- Composites (9)
- Computer Science (17)
- Coronavirus (4)
- Critical Materials (13)
- Cybersecurity (4)
- Decarbonization (7)
- Exascale Computing (2)
- Frontier (3)
- Fusion (7)
- Grid (5)
- High-Performance Computing (4)
- Irradiation (1)
- Isotopes (13)
- ITER (1)
- Machine Learning (5)
- Materials (73)
- Materials Science (78)
- Mathematics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (3)
- Net Zero (1)
- Neutron Science (33)
- Nuclear Energy (16)
- Physics (29)
- Polymers (17)
- Quantum Computing (3)
- Quantum Science (11)
- Renewable Energy (1)
- Security (2)
- Simulation (1)
- Space Exploration (2)
- Sustainable Energy (13)
- Transformational Challenge Reactor (3)
- Transportation (14)
Media Contacts
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
In the race to identify solutions to the COVID-19 pandemic, researchers at the Department of Energy’s Oak Ridge National Laboratory are joining the fight by applying expertise in computational science, advanced manufacturing, data science and neutron science.

Energy storage startup SPARKZ Inc. has exclusively licensed five battery technologies from the Department of Energy’s Oak Ridge National Laboratory designed to eliminate cobalt metal in lithium-ion batteries. The advancement is aimed at accelerating the production of electric vehicles and energy storage solutions for the power grid.
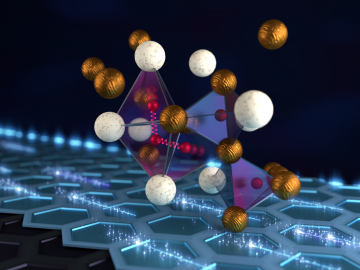
An international team of researchers has discovered the hydrogen atoms in a metal hydride material are much more tightly spaced than had been predicted for decades — a feature that could possibly facilitate superconductivity at or near room temperature and pressure.

The formation of lithium dendrites is still a mystery, but materials engineers study the conditions that enable dendrites and how to stop them.

Students often participate in internships and receive formal training in their chosen career fields during college, but some pursue professional development opportunities even earlier.
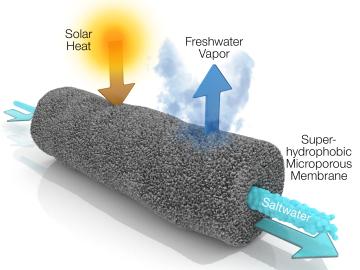
A new method developed at Oak Ridge National Laboratory improves the energy efficiency of a desalination process known as solar-thermal evaporation.
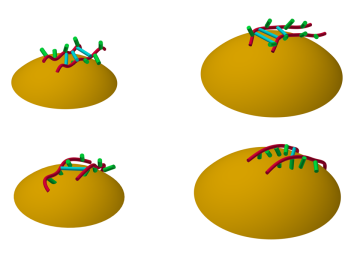
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
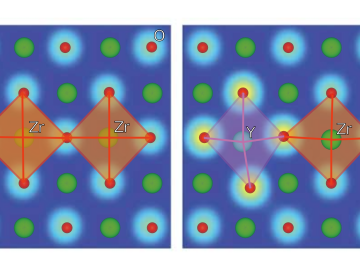
Ionic conduction involves the movement of ions from one location to another inside a material. The ions travel through point defects, which are irregularities in the otherwise consistent arrangement of atoms known as the crystal lattice. This sometimes sluggish process can limit the performance and efficiency of fuel cells, batteries, and other energy storage technologies.


