Filter News
Area of Research
- (-) Materials (21)
- Advanced Manufacturing (1)
- Biology and Environment (3)
- Clean Energy (17)
- Fusion and Fission (6)
- Fusion Energy (7)
- Materials for Computing (6)
- National Security (1)
- Neutron Science (2)
- Nuclear Science and Technology (11)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Supercomputing (3)
News Topics
- (-) Advanced Reactors (4)
- (-) Polymers (17)
- 3-D Printing/Advanced Manufacturing (23)
- Artificial Intelligence (9)
- Big Data (2)
- Bioenergy (11)
- Biology (4)
- Biomedical (7)
- Buildings (5)
- Chemical Sciences (32)
- Clean Water (3)
- Climate Change (5)
- Composites (9)
- Computer Science (17)
- Coronavirus (4)
- Critical Materials (13)
- Cybersecurity (4)
- Decarbonization (7)
- Energy Storage (34)
- Environment (15)
- Exascale Computing (2)
- Frontier (3)
- Fusion (7)
- Grid (5)
- High-Performance Computing (4)
- Irradiation (1)
- Isotopes (13)
- ITER (1)
- Machine Learning (5)
- Materials (73)
- Materials Science (78)
- Mathematics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (3)
- Net Zero (1)
- Neutron Science (33)
- Nuclear Energy (16)
- Partnerships (11)
- Physics (29)
- Quantum Computing (3)
- Quantum Science (11)
- Renewable Energy (1)
- Security (2)
- Simulation (1)
- Space Exploration (2)
- Summit (2)
- Sustainable Energy (13)
- Transformational Challenge Reactor (3)
- Transportation (14)
Media Contacts
Oak Ridge National Laboratory scientists have discovered a cost-effective way to significantly improve the mechanical performance of common polymer nanocomposite materials.
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.

Scientists at the Department of Energy Manufacturing Demonstration Facility at ORNL have their eyes on the prize: the Transformational Challenge Reactor, or TCR, a microreactor built using 3D printing and other new approaches that will be up and running by 2023.
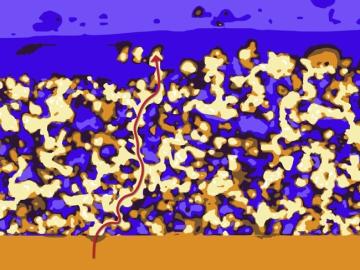
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
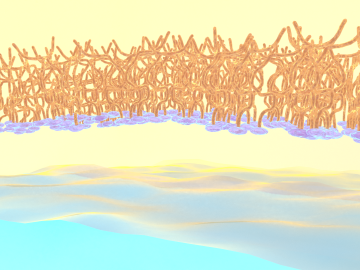
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.

Using additive manufacturing, scientists experimenting with tungsten at Oak Ridge National Laboratory hope to unlock new potential of the high-performance heat-transferring material used to protect components from the plasma inside a fusion reactor. Fusion requires hydrogen isotopes to reach millions of degrees.
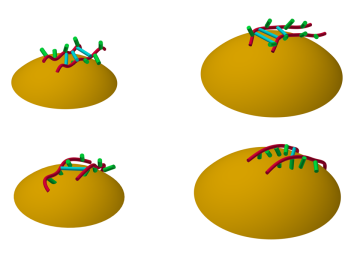
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

Carbon fiber composites—lightweight and strong—are great structural materials for automobiles, aircraft and other transportation vehicles. They consist of a polymer matrix, such as epoxy, into which reinforcing carbon fibers have been embedded. Because of differences in the mecha...

Oak Ridge National Laboratory scientists have developed a crucial component for a new kind of low-cost stationary battery system utilizing common materials and designed for grid-scale electricity storage. Large, economical electricity storage systems can benefit the nation’s grid ...