Filter News
Area of Research
- (-) Materials (122)
- Advanced Manufacturing (6)
- Biology and Environment (118)
- Biology and Soft Matter (1)
- Clean Energy (111)
- Climate and Environmental Systems (6)
- Computational Biology (2)
- Computational Engineering (2)
- Computer Science (3)
- Electricity and Smart Grid (1)
- Fuel Cycle Science and Technology (1)
- Functional Materials for Energy (1)
- Fusion and Fission (33)
- Fusion Energy (11)
- Isotope Development and Production (1)
- Isotopes (26)
- Materials Characterization (1)
- Materials for Computing (21)
- Materials Under Extremes (1)
- Mathematics (1)
- National Security (19)
- Neutron Science (43)
- Nuclear Science and Technology (40)
- Nuclear Systems Modeling, Simulation and Validation (1)
- Supercomputing (115)
- Transportation Systems (1)
News Topics
- (-) Clean Water (3)
- (-) Climate Change (5)
- (-) Coronavirus (4)
- (-) Environment (15)
- (-) Isotopes (13)
- (-) Materials Science (78)
- (-) Nuclear Energy (16)
- (-) Polymers (17)
- (-) Quantum Computing (3)
- (-) Simulation (1)
- (-) Summit (2)
- 3-D Printing/Advanced Manufacturing (23)
- Advanced Reactors (4)
- Artificial Intelligence (9)
- Big Data (2)
- Bioenergy (11)
- Biology (4)
- Biomedical (7)
- Buildings (5)
- Chemical Sciences (32)
- Composites (9)
- Computer Science (17)
- Critical Materials (13)
- Cybersecurity (4)
- Decarbonization (7)
- Energy Storage (34)
- Exascale Computing (2)
- Frontier (3)
- Fusion (7)
- Grid (5)
- High-Performance Computing (4)
- Irradiation (1)
- ITER (1)
- Machine Learning (5)
- Materials (73)
- Mathematics (1)
- Microscopy (27)
- Molten Salt (3)
- Nanotechnology (39)
- National Security (3)
- Net Zero (1)
- Neutron Science (33)
- Partnerships (11)
- Physics (29)
- Quantum Science (11)
- Renewable Energy (1)
- Security (2)
- Space Exploration (2)
- Sustainable Energy (13)
- Transformational Challenge Reactor (3)
- Transportation (14)
Media Contacts

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.
Researchers used neutron scattering at Oak Ridge National Laboratory’s Spallation Neutron Source to investigate the effectiveness of a novel crystallization method to capture carbon dioxide directly from the air.

Scientists have tested a novel heat-shielding graphite foam, originally created at Oak Ridge National Laboratory, at Germany’s Wendelstein 7-X stellarator with promising results for use in plasma-facing components of fusion reactors.

OAK RIDGE, Tenn., Feb. 8, 2019—The Department of Energy’s Oak Ridge National Laboratory has named Sean Hearne director of the Center for Nanophase Materials Sciences. The center is a DOE Office of Science User Facility that brings world-leading resources and capabilities to the nanoscience resear...
Scientists at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have developed a process that could remove CO2 from coal-burning power plant emissions in a way that is similar to how soda lime works in scuba diving rebreathers. Their research, published January 31 in...

OAK RIDGE, Tenn., Jan. 31, 2019—A new electron microscopy technique that detects the subtle changes in the weight of proteins at the nanoscale—while keeping the sample intact—could open a new pathway for deeper, more comprehensive studies of the basic building blocks of life.

Jon Poplawsky, a materials scientist at the Department of Energy’s Oak Ridge National Laboratory, develops and links advanced characterization techniques that improve our ability to see and understand atomic-scale features of diverse materials

Carbon fiber composites—lightweight and strong—are great structural materials for automobiles, aircraft and other transportation vehicles. They consist of a polymer matrix, such as epoxy, into which reinforcing carbon fibers have been embedded. Because of differences in the mecha...
Physicists turned to the “doubly magic” tin isotope Sn-132, colliding it with a target at Oak Ridge National Laboratory to assess its properties as it lost a neutron to become Sn-131.
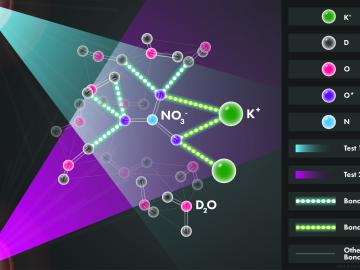
Scientists at the Department of Energy’s Oak Ridge National Laboratory used neutrons, isotopes and simulations to “see” the atomic structure of a saturated solution and found evidence supporting one of two competing hypotheses about how ions come


