
Filter News
Area of Research
News Topics
- (-) Nanotechnology (60)
- (-) Polymers (33)
- (-) Statistics (3)
- 3-D Printing/Advanced Manufacturing (120)
- Advanced Reactors (34)
- Artificial Intelligence (91)
- Big Data (53)
- Bioenergy (91)
- Biology (98)
- Biomedical (58)
- Biotechnology (22)
- Buildings (57)
- Chemical Sciences (63)
- Clean Water (29)
- Climate Change (99)
- Composites (26)
- Computer Science (187)
- Coronavirus (46)
- Critical Materials (25)
- Cybersecurity (35)
- Decarbonization (79)
- Education (4)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (108)
- Environment (194)
- Exascale Computing (37)
- Fossil Energy (5)
- Frontier (42)
- Fusion (54)
- Grid (62)
- High-Performance Computing (84)
- Hydropower (11)
- Irradiation (3)
- Isotopes (53)
- ITER (7)
- Machine Learning (47)
- Materials (143)
- Materials Science (139)
- Mathematics (7)
- Mercury (12)
- Microelectronics (3)
- Microscopy (51)
- Molten Salt (8)
- National Security (61)
- Net Zero (13)
- Neutron Science (131)
- Nuclear Energy (107)
- Partnerships (43)
- Physics (61)
- Quantum Computing (34)
- Quantum Science (69)
- Renewable Energy (2)
- Security (24)
- Simulation (47)
- Software (1)
- Space Exploration (25)
- Summit (57)
- Sustainable Energy (125)
- Transformational Challenge Reactor (7)
- Transportation (97)
Media Contacts
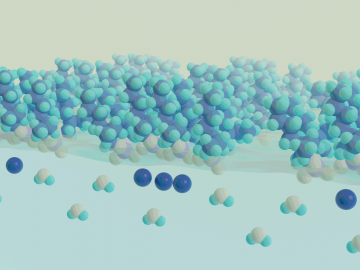
Real-time measurements captured by researchers at ORNL provide missing insight into chemical separations to recover cobalt, a critical raw material used to make batteries and magnets for modern technologies.
From materials science and earth system modeling to quantum information science and cybersecurity, experts in many fields run simulations and conduct experiments to collect the abundance of data necessary for scientific progress.

Five researchers at the Department of Energy’s Oak Ridge National Laboratory have been named ORNL Corporate Fellows in recognition of significant career accomplishments and continued leadership in their scientific fields.
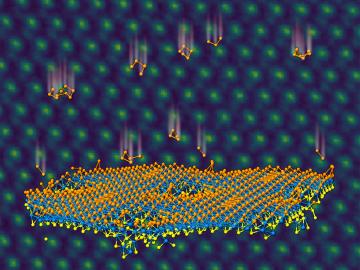
An ORNL team used a simple process to implant atoms precisely into the top layers of ultra-thin crystals, yielding two-sided structures with different chemical compositions.

ORNL welcomed six technology innovators to join the fourth cohort of Innovation Crossroads, the Southeast’s only entrepreneurial research and development program based at a U.S. Department of Energy national laboratory.
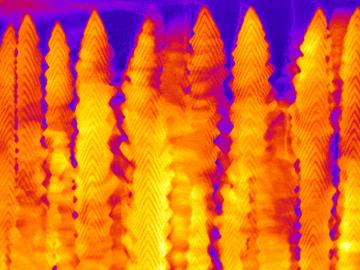
A team led by the Department of Energy’s Oak Ridge National Laboratory synthesized a tiny structure with high surface area and discovered how its unique architecture drives ions across interfaces to transport energy or information.

Matthew R. Ryder, a researcher at the Department of Energy’s Oak Ridge National Laboratory, has been named the 2020 Foresight Fellow in Molecular-Scale Engineering.
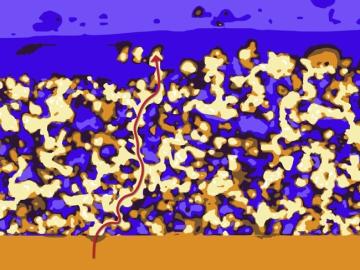
Oak Ridge National Laboratory researchers have developed a thin film, highly conductive solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries.
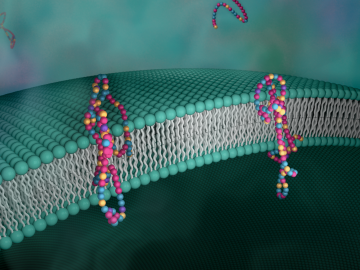
Biological membranes, such as the “walls” of most types of living cells, primarily consist of a double layer of lipids, or “lipid bilayer,” that forms the structure, and a variety of embedded and attached proteins with highly specialized functions, including proteins that rapidly and selectively transport ions and molecules in and out of the cell.
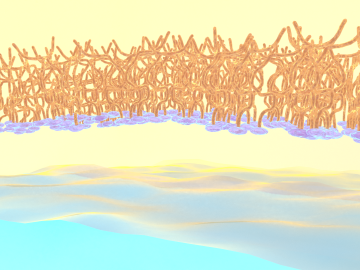
OAK RIDGE, Tenn., Feb. 27, 2020 — Researchers at Oak Ridge National Laboratory and the University of Tennessee achieved a rare look at the inner workings of polymer self-assembly at an oil-water interface to advance materials for neuromorphic computing and bio-inspired technologies.


