
Filter News
Area of Research
News Topics
- (-) Clean Water (29)
- (-) Critical Materials (25)
- (-) Molten Salt (8)
- (-) Polymers (33)
- 3-D Printing/Advanced Manufacturing (119)
- Advanced Reactors (34)
- Artificial Intelligence (91)
- Big Data (53)
- Bioenergy (91)
- Biology (98)
- Biomedical (58)
- Biotechnology (22)
- Buildings (57)
- Chemical Sciences (63)
- Climate Change (99)
- Composites (26)
- Computer Science (187)
- Coronavirus (46)
- Cybersecurity (35)
- Decarbonization (79)
- Education (4)
- Element Discovery (1)
- Emergency (2)
- Energy Storage (108)
- Environment (194)
- Exascale Computing (37)
- Fossil Energy (5)
- Frontier (42)
- Fusion (54)
- Grid (62)
- High-Performance Computing (84)
- Hydropower (11)
- Irradiation (3)
- Isotopes (53)
- ITER (7)
- Machine Learning (47)
- Materials (143)
- Materials Science (139)
- Mathematics (7)
- Mercury (12)
- Microelectronics (3)
- Microscopy (51)
- Nanotechnology (60)
- National Security (61)
- Net Zero (13)
- Neutron Science (131)
- Nuclear Energy (107)
- Partnerships (43)
- Physics (61)
- Quantum Computing (34)
- Quantum Science (69)
- Renewable Energy (2)
- Security (24)
- Simulation (47)
- Software (1)
- Space Exploration (25)
- Statistics (3)
- Summit (57)
- Sustainable Energy (125)
- Transformational Challenge Reactor (7)
- Transportation (97)
Media Contacts

The National Alliance for Water Innovation, a partnership of the Department of Energy’s Oak Ridge National Laboratory, other national labs, university and private sector partners, has been awarded a five-year, $100 million Energy-Water Desalination Hub by DOE to address water security issues in the United States.
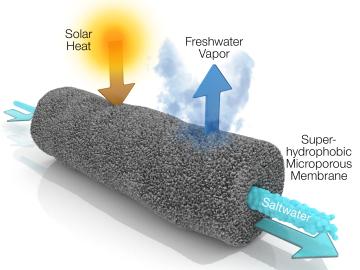
A new method developed at Oak Ridge National Laboratory improves the energy efficiency of a desalination process known as solar-thermal evaporation.
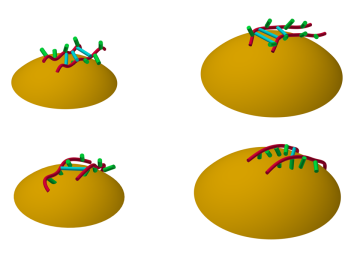
A team of researchers at Oak Ridge National Laboratory have demonstrated that designed synthetic polymers can serve as a high-performance binding material for next-generation lithium-ion batteries.

Researchers at the Department of Energy’s Oak Ridge National Laboratory, Pacific Northwest National Laboratory and Washington State University teamed up to investigate the complex dynamics of low-water liquids that challenge nuclear waste processing at federal cleanup sites.
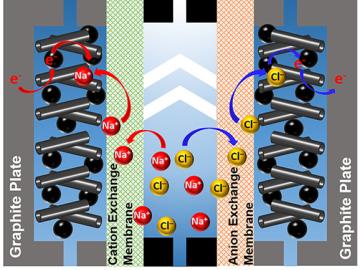
A team of scientists led by Oak Ridge National Laboratory used carbon nanotubes to improve a desalination process that attracts and removes ionic compounds such as salt from water using charged electrodes.

Vera Bocharova at the Department of Energy’s Oak Ridge National Laboratory investigates the structure and dynamics of soft materials—polymer nanocomposites, polymer electrolytes and biological macromolecules—to advance materials and technologies for energy, medicine and other applications.

Oak Ridge National Laboratory scientists analyzed more than 50 years of data showing puzzlingly inconsistent trends about corrosion of structural alloys in molten salts and found one factor mattered most—salt purity.

Scientists from Oak Ridge National Laboratory performed a corrosion test in a neutron radiation field to support the continued development of molten salt reactors.

Carbon fiber composites—lightweight and strong—are great structural materials for automobiles, aircraft and other transportation vehicles. They consist of a polymer matrix, such as epoxy, into which reinforcing carbon fibers have been embedded. Because of differences in the mecha...
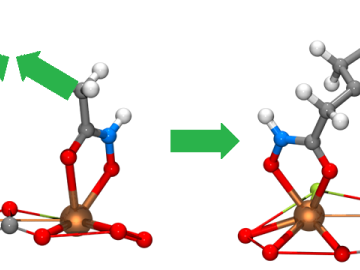
Scientists from the Critical Materials Institute used the Titan supercomputer and Eos computing cluster at ORNL to analyze designer molecules that could increase the yield of rare earth elements found in bastnaesite, an important mineral


